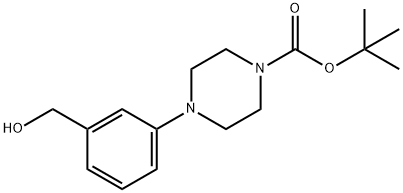
tert-butyl 4-[3-(hydroxymethyl)phenyl]piperazine-1-carboxylate synthesis
- Product Name:tert-butyl 4-[3-(hydroxymethyl)phenyl]piperazine-1-carboxylate
- CAS Number:261925-88-6
- Molecular formula:C16H24N2O3
- Molecular Weight:292.37
![4-[3-(METHOXYCARBONYL)PHENYL]-1-PIPERAZINECARBOXYLIC ACID, 1,1-DIMETHYLETHYL ESTER](/CAS/GIF/179003-10-2.gif)
179003-10-2
13 suppliers
inquiry
![tert-butyl 4-[3-(hydroxymethyl)phenyl]piperazine-1-carboxylate](/CAS/GIF/261925-88-6.gif)
261925-88-6
19 suppliers
$502.88/5MG
Yield:261925-88-6 98.6%
Reaction Conditions:
with lithium borohydride in tetrahydrofuran at 20;Inert atmosphere;Cooling with ice;
Steps:
217 21 7C. tert-Butyl 4-(3-(hydroxymethyl)phenyl)piperazine- 1 -carboxylate
217B (171 mg, 0.534 mmol) was dissolved in THF (5.3 mL) and cooled in an ice bath with stirring under argon. A 2M solution of LiBH4 in THF (0.53 mL, 1.1 mmol)was added dropwise. The resulting pale yellow solution was stirred for 1 h in an ice bath, then overnight at a The reaction mixture was heated to reflux for 1 h, then cooled to 0 °C and quenched with 1M HC1 to pH 1. The mixture was stirred for 30 mm, then the pH was adjusted to 9-10 with solid K2C03. The mixture was extracted with EtOAc (2x). Thecombined extracts were washed with brine, dried over Na2SO4, filtered and evaporated togive 217C (154 mg, 98.6%) as a white solid. MS(ESI) m/z 292.9 (M+H).
References:
WO2017/40449,2017,A1 Location in patent:Paragraph 00524
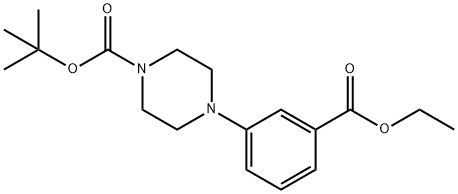
261925-94-4
40 suppliers
$63.70/1g
![tert-butyl 4-[3-(hydroxymethyl)phenyl]piperazine-1-carboxylate](/CAS/GIF/261925-88-6.gif)
261925-88-6
19 suppliers
$502.88/5MG
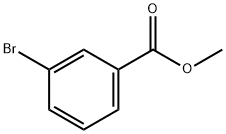
618-89-3
267 suppliers
$32.00/5g
![tert-butyl 4-[3-(hydroxymethyl)phenyl]piperazine-1-carboxylate](/CAS/GIF/261925-88-6.gif)
261925-88-6
19 suppliers
$502.88/5MG

57260-71-6
741 suppliers
$5.00/5g
![tert-butyl 4-[3-(hydroxymethyl)phenyl]piperazine-1-carboxylate](/CAS/GIF/261925-88-6.gif)
261925-88-6
19 suppliers
$502.88/5MG
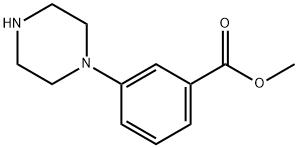
179003-08-8
20 suppliers
inquiry
![tert-butyl 4-[3-(hydroxymethyl)phenyl]piperazine-1-carboxylate](/CAS/GIF/261925-88-6.gif)
261925-88-6
19 suppliers
$502.88/5MG