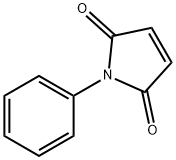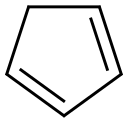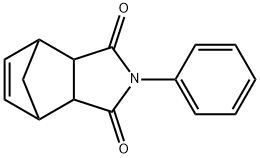
2-phenyl-3a,4,7,7a-tetrahydro-1H-4,7-methanoisoindole-1,3(2H)-dione synthesis
- Product Name:2-phenyl-3a,4,7,7a-tetrahydro-1H-4,7-methanoisoindole-1,3(2H)-dione
- CAS Number:26234-46-8
- Molecular formula:C15H13NO2
- Molecular Weight:239.27
Yield:26234-46-8 82%
Reaction Conditions:
Stage #1: N-phenyl-maleimide;bi(cyclopentadiene)with 4-(1-methylpyrrolidin-1-ium)methyl-2,2-dimethyl-1,3-dioxolanetrifluoro-methansulfonamide at 160; for 23 h;Inert atmosphere;Diels-Alder Cycloaddition;
Stage #2: with hydrogenchloride in water at 50; for 19 h;Inert atmosphere;Diels-Alder Cycloaddition;
Steps:
Diels-Alder reaction of maleimide in [PGA][TFSA] 5b:
Dicyclopentadiene (200.6mg, 1.5 mmol) and N-phenylmaleimide (263.0 mg, 1.5 mmol) were added to ionic liquid 5b (779.4 mg, 1.6mmol), and the reaction mixture was stirred at 160 °C for 23 h.After cooled, aqueous HCl (1 M, 10 mL) was added to the reaction mixture and stirred at 50 °C for 19 h. Water (10 mL) was added to the mixture and the resulting solution was extracted with CH2Cl2 (3 × 20 mL). The organic phases were combined and dried over Na2SO4. After filtered, the filtrate was concentrated in vacuo to give adduct 2-phenyl-3a,4,7,7a-tetrahydro-1H-4,7-methanoisoindole-1,3(2H)-dione 7 in 82% yield(298.6 mg, 1.25mmol).White solid; mp 138 °C; 1H NMR (500 MHz, CDCl3) d 7.42 (t, J = 7.6 Hz, 2H), 7.35 (t,J = 7.4 Hz, 1H), 7.12 (d, J = 7.8 Hz, 2H), 6.25 (t, J = 1.6 Hz, 2H), 3.49 (dt, J = 4.5, 2.6Hz, 2H), 3.42 (dd, J = 2.9, 1.6 Hz, 2H), 1.77 (dt, J = 8.8, 1.6 Hz, 1H), 1.60 (d, J = 8.8Hz, 1H); 13C NMR (125 MHz, CDCl3) d 177.0, 134.7, 131.9, 129.2, 128.7, 126.8, 52.4,45.9, 45.6; HRMS: Found (ESI): [M+H]+ 240.1028 C15H14NO2, requires 240.1025.Concentration of aqueous solution gave ionic liquid 6b in 92% yield (661.1 mg,1.5mmol).
References:
Kamimura, Akio;Shiramatsu, Yuto;Murata, Kengo;Kawamoto, Takuji [Chemistry Letters,2018,vol. 47,# 8,p. 1079 - 1081] Location in patent:supporting information
968-39-8
21 suppliers
$55.00/500mg

26234-46-8
13 suppliers
inquiry