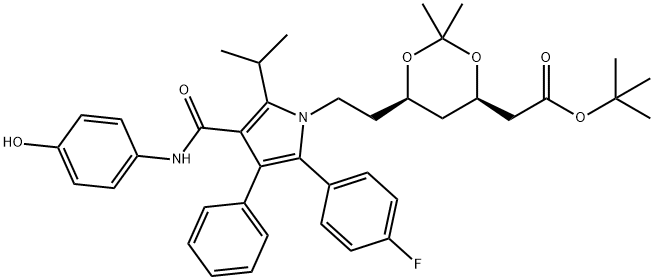
tert-butyl 2-((4R,6R)-6-(2-(2-(4- fluorophenyl)-4-(4- hydroxyphenylcarbamoyl)-5-isopropyl-3- phenyl-1H-pyrrol-1-yl)ethyl)-2,2- dimethyl-1,3-dioxan-4-yl)acetate synthesis
- Product Name:tert-butyl 2-((4R,6R)-6-(2-(2-(4- fluorophenyl)-4-(4- hydroxyphenylcarbamoyl)-5-isopropyl-3- phenyl-1H-pyrrol-1-yl)ethyl)-2,2- dimethyl-1,3-dioxan-4-yl)acetate
- CAS Number:265989-36-4
- Molecular formula:C40H47FN2O6
- Molecular Weight:670.81
![(6-{2-[3-(4-Benzyloxy-phenylcarbamoyl)-5-(4-fluoro-phenyl)-2-isopropyl-4-phenyl-pyrrol-1-yl]-ethyl}-2,2-dimethyl-[1,3]-dioxane-4-yl)-acetic Acid, tert-Butyl Ester](/CAS2/GIF/163217-68-3.gif)
163217-68-3
9 suppliers
inquiry

265989-36-4
17 suppliers
$95.00/2mg
Yield:265989-36-4 89.8%
Reaction Conditions:
with palladium on activated charcoal;hydrogen in methanol at 50; under 2585.81 Torr; for 20 h;
Steps:
5 Step 5: Synthesis of tert-butyl 2-((4R,6R)-6-(2-(2-(4-fluorophenyl)-4-((4-hydroxyphenyl)carbamoyl)-5-isopropyl-3-phenyl-1H-pyrrol-1-yl)ethyl)-2,2-dimethyl-1,3-dioxan-4-yl)acetate (9)
In MeOH (500 mL) tert-Butyl 2-((4R,6R)-6-(2-(3-((4-(benzyloxy)phenyl)carbamoyl)-5-(4-fluorophenyl)-2-isopropyl-4 phenyl-1H-pyrrol-1-yl)ethyl)-2,2-dimethyl-1,3-dioxan-4-yl)acetate (4.8 g, 6.31 mmol) add Pd/C (0.8 g, 6.31 mmol, 10% purity) to the mixture, The resulting mixture was stirred at 50° C. under H2 (50 psi) for 20 hours. LCMS showed the main peak of the desired mass, The starting material was consumed. The mixture was filtered through a pad of Celite and the filtrate was concentrated. The residue was purified by silica gel column chromatography (100% EA) to obtain the title compound (3.8 g, 5.66 mmol, 89.80% yield) as a white solid.
References:
KR102160377,2020,B1 Location in patent:Paragraph 0133; 0190-0194
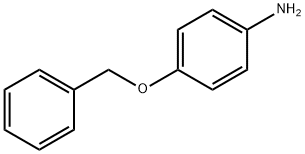
6373-46-2
159 suppliers
$6.00/1g

265989-36-4
17 suppliers
$95.00/2mg

265989-30-8
18 suppliers
inquiry

265989-36-4
17 suppliers
$95.00/2mg

163217-66-1
12 suppliers
inquiry

265989-36-4
17 suppliers
$95.00/2mg
![2-[2-(4-Fluorophenyl)-2-oxo-1-phenyl-ethyl]-4-methyl-3-oxo-pentanoic Acid, (4-Benzyloxy-phenyl)-amide](/CAS2/GIF/163217-67-2.gif)
163217-67-2
8 suppliers
inquiry

265989-36-4
17 suppliers
$95.00/2mg