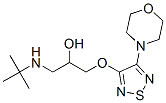
(+)-1-(tert-butylamino)-3-[(4-morpholino-1,2,5-thiadiazol-3-yl)oxy]propan-2-ol synthesis
- Product Name:(+)-1-(tert-butylamino)-3-[(4-morpholino-1,2,5-thiadiazol-3-yl)oxy]propan-2-ol
- CAS Number:26839-76-9
- Molecular formula:C13H24N4O3S
- Molecular Weight:316.4196
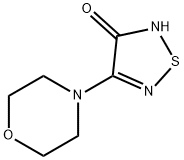
30165-97-0
111 suppliers
$87.00/250mg
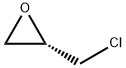
67843-74-7
478 suppliers
$10.00/1g
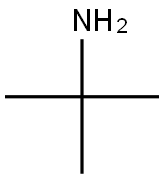
75-64-9
422 suppliers
$10.00/10g
![(+)-1-(tert-butylamino)-3-[(4-morpholino-1,2,5-thiadiazol-3-yl)oxy]propan-2-ol](/CAS/GIF/26839-76-9.gif)
26839-76-9
31 suppliers
$150.00/10mg
Yield: 98.6 % ee
Reaction Conditions:
Stage #1:3-hydroxy-4-(N-morpholino)-1,2,5-thiadiazole;(S)-epichlorohydrin with sodium carbonate in tetrahydrofuran at 60 - 65; for 14.5 h;
Stage #2:tert-butylamine at 44 - 46; for 3 h;Product distribution / selectivity;
Steps:
4
Example 4 Preparation of R-(+)-3-morpholino-4-(3-tert-butylamino-2-hydroxypropoxy)-1,2,5-thiadiazole30.0 grams of 3-hydroxy-4-morpholino-1,2,5-thiadiazole and 21.8 ml of S-(+)-epichlorohydrin is mixed in the present of 80.0 ml of tetrahydrofuran and 6.4 grams of sodium carbonate. The mixture is heated to and maintained at 60° C.65° C., and, in the meantime, stirred for 14.5 hours. Excess S-(+)-epichlorohydrin is removed by concentration in vacuo under a low pressure about 750 mmHg and at the temperature about 80° C. The oily residue is then obtained. The obtained oily residue is mixed with 400.0 ml of tert-butylamine, followed by being heating to and maintaining at 44° C.46° C. and, in the meantime, being stirring for 3 hours. Excess tert-butylamine is removed by concentration in vacuo under a low pressure about 720 mmHg and at the temperature about 40° C. The obtained oily residue is R-(+)-3-morpholino-4-(3-tert-butylamino-2-hydroxypropoxy)-1,2,5-thiadiazole. The purity of the final product obtained is 99.3% in R-(+)-form and 0.7% in S-(-)-form by HPLC. Thus, the e.e. value of the R-(+)-3-morpholino-4-(3-tert-butylamino-2-hydroxypropoxy)-1,2,5-thiadiazole obtained from this preparation is 98.6%.
References:
FAN, Chin-Tsai;Hsiao, Chen-Ming;Hsieh, Rong-Bin US2011/92507, 2011, A1 Location in patent:Page/Page column 5-6