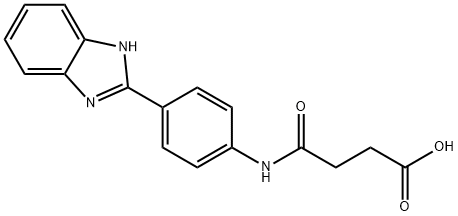
4-[[4-(1H-benzimidazol-2-yl)phenyl]amino]-4-keto-butyric acid synthesis
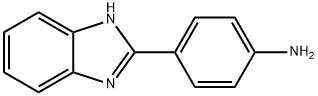
- Product Name:4-[[4-(1H-benzimidazol-2-yl)phenyl]amino]-4-keto-butyric acid
- CAS Number:27031-00-1
- Molecular formula:C17H15N3O3
- Molecular Weight:309.32
Yield:27031-00-1 71%
Reaction Conditions:
in tetrahydrofuran at 160 - 170;Molecular sieve;Microwave irradiation;
Steps:
2 4.2.8. General procedure for the synthesis of acylated derivatives (4-6, 8-11, 13-16, 18-21, 23-26, 28-31, 33)procedure B
General procedure: The synthesis followed the literature procedure. To a well stirredsolution of the corresponding 4-(sub:-1H-benzo[d]imidazol-2-yl)aniline/4-(3H-imidazo[4,5-b]pyridin-2-yl)aniline (3a-f)(1 equiv) in anhydrous tetrahydrofuran (0.15 M) with 4 ? molecularsieves in an appropriate sized microwave vial; was added thecorresponding anhydride (1 equiv) at room temperature. The reactionmixture was then irradiated in Biotage microwave initiatorwith stirring at 160-170 C for about 20-30 min (monitored byTLC and LCMS for completion). The solvent was removed undervacuum, diluted with water and basified with solid sodium bicarbonateto a pH of 9. The aqueous layer was further washed withdichloromethane, acidified with 2 N HCl to a pH of 2, and extractedrepeatedly with ethyl acetate. The combined organic layer wasthen dried over anhydrous sodium sulfate and concentrated underreduced pressure. The residue obtained was further recrystallizedfrom ethanol to afford the desired product in good yield and purityas described below.
References:
Janupally, Renuka;Jeankumar, Variam Ullas;Bobesh, Karyakulam Andrews;Soni, Vijay;Devi, Parthiban Brindha;Pulla, Venkat Koushik;Suryadevara, Priyanka;Chennubhotla, Keerthana Sharma;Kulkarni, Pushkar;Yogeeswari, Perumal;Sriram, Dharmarajan [Bioorganic and Medicinal Chemistry,2014,vol. 22,# 21,p. 5970 - 5987]

150-13-0
886 suppliers
$5.00/25g
![4-[[4-(1H-benzimidazol-2-yl)phenyl]amino]-4-keto-butyric acid](/CAS/GIF/27031-00-1.gif)
27031-00-1
16 suppliers
inquiry
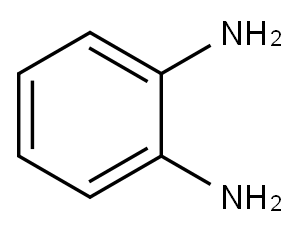
95-54-5
499 suppliers
$9.00/1g
![4-[[4-(1H-benzimidazol-2-yl)phenyl]amino]-4-keto-butyric acid](/CAS/GIF/27031-00-1.gif)
27031-00-1
16 suppliers
inquiry