[3-(4-CHLOROPHENYL)-2-OXIRANYL](4-METHOXYPHENYL)METHANONE synthesis
- Product Name:[3-(4-CHLOROPHENYL)-2-OXIRANYL](4-METHOXYPHENYL)METHANONE
- CAS Number:27547-08-6
- Molecular formula:C16H13ClO3
- Molecular Weight:288.73

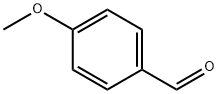
6552-68-7
19 suppliers
$50.00/500mg
METHANONE](/StructureFile/ChemBookStructure5/GIF/CB9331248.gif)
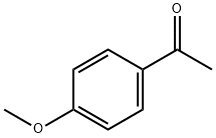
27547-08-6
6 suppliers
inquiry
Yield:-
Reaction Conditions:
with dihydrogen peroxide at 0 - 2; for 1.5 h;Green chemistry;
Steps:
General procedure: A 30 wt % aq NaOH solution (1 mL) was added to amixture of substituted benzaldehyde (5 mmol), substituted acetophenone(5 mmol) and methanol (10 mL), and stirred at room temperature for 1.5 h. Thereaction mixture was heated to dissolve the solid and a 30% hydrogen peroxidesolution (1 mL) was added. Then, the reaction was stirred between 0 and 2 Cfor 1.5 h. The final chalcone epoxides were recovered by vacuum filtration.
References:
Ngo, Dalyna;Kalala, Mbelu;Hogan, Victoria;Manchanayakage, Renuka [Tetrahedron Letters,2014,vol. 55,# 32,p. 4496 - 4500]