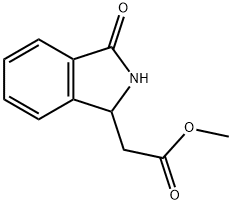
(3-Oxo-2,3-dihydro-1H-isoindol-1-yl)acetic acid methyl ester synthesis
- Product Name:(3-Oxo-2,3-dihydro-1H-isoindol-1-yl)acetic acid methyl ester
- CAS Number:28488-99-5
- Molecular formula:C11H11NO3
- Molecular Weight:205.21
Yield:28488-99-5 98%
Reaction Conditions:
with sulfuric acid at 20; for 16.0833 h;
Steps:
6.A
Example 6 6-{2-[2-(4-Fluoro-benzyl)-3-oxo-2,3-dihydro-1H-isoindol-1-yl]-acetylamino}-nicotinic acid A. (3-Oxo-2,3-dihydro-1H-isoindol-1-yl)-acetic acid methyl ester; To a solution of (3-oxo-2,3-dihydro-1H-isoindol-1-yl)-acetic acid (2.0 g, 10.4 mmol) in 100 mL methanol was added 2 mL conc. H2SO4 over 5 min. The mixture was stirred at room temperature for 16 h. Solvent was evaporated, the crude oil was dissolved in 200 mL EtOAc, then washed with 50 mL sat'd NaHCO3 and 2×50 mL water. The organic phase was dried (Na2SO4), filtered, and solvent was evaporated. The title compound was isolated as a yellow oil (2.1 g, 98%).
References:
US2007/99930,2007,A1 Location in patent:Page/Page column 31
![[1(3H)-ISOINDOLINONE-3-YL]ACETIC ACID](/CAS/GIF/3849-22-7.gif)
3849-22-7
35 suppliers
$45.00/10mg

75-09-2
1197 suppliers
$10.00/25ML

74-88-4
340 suppliers
$15.00/10g

28488-99-5
11 suppliers
inquiry
![[1(3H)-ISOINDOLINONE-3-YL]ACETIC ACID](/CAS/GIF/3849-22-7.gif)
3849-22-7
35 suppliers
$45.00/10mg

28488-99-5
11 suppliers
inquiry
7468-67-9
238 suppliers
$13.00/5g

28488-99-5
11 suppliers
inquiry