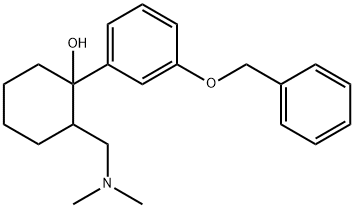
1-(3-(Benzyloxy)phenyl)-2-((diMethylaMino)Methyl)cyclohexanol synthesis
- Product Name:1-(3-(Benzyloxy)phenyl)-2-((diMethylaMino)Methyl)cyclohexanol
- CAS Number:2914-85-4
- Molecular formula:C22H29NO2
- Molecular Weight:339.47
Yield:2914-85-4 15.75 g
Reaction Conditions:
Stage #1: 3-(benzyloxy)phenyl bromidewith iodine;magnesium in tetrahydrofuran;diethyl ether; for 1 h;Inert atmosphere;Reflux;
Stage #2: 2-[(dimethylamino)methyl]cyclohexanone in tetrahydrofuran;diethyl ether at 0 - 20; for 4 h;Inert atmosphere;
Steps:
3 Preparation of 1-(3-(benzyloxy)phenyl)-2-((dimethylamino)methyl)cyclohexan-1-ol 4
To a suspension of magnesium (6 g, 0.25 mol, 3 eq) in dry diethyl ether (30 ml) was added iodine crystal followed by a solution of 3-(benzyloxy)phenyl bromide 3 (22 g, 83.7 mmol) in dry tetrahydrofuran (50 ml) dropwise at a rate (once reaction has initiated), under a nitrogen atmosphere, maintaining a gentle reflux. The resulting mixture was heated at reflux for 1 hour. The mixture was cooled to 0 °C. and a solution of 2-[(dimethylamino)methyl]cyclohexan-1-one 2 (13 g, 83.7 mmol, 1 eq) in dry tetrahydrofuran (50 ml) was added dropwise. The reaction mixture was stirred for 4 hours at RT (room temperature). The reaction mixture was cooled at 0° C and an aqueous solution of ammonium chloride was added dropwise and the resulting suspension was stirred at room temperature overnight. The mixture was filtered through a pad of Celite and washed with ethyl acetate (3×200 ml). The extracts were combined, washed with brine, dried over sodium sulfate, filtered and evaporated to dryness. The residue obtained was purified by column chromatography (silica gel; 0-50% ethyl acetate in hexane) to give the title compound (15.75 g) 4 as a yellow oil.
References:
US2017/176476,2017,A1 Location in patent:Paragraph 0009; 0080

591-20-8
334 suppliers
$10.00/1g

2914-85-4
6 suppliers
inquiry
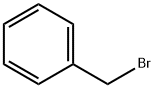
100-39-0
424 suppliers
$10.00/10g

2914-85-4
6 suppliers
inquiry
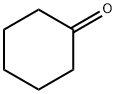
108-94-1
528 suppliers
$12.00/50g

2914-85-4
6 suppliers
inquiry

![Cyclohexanone, 2-[(dimethylamino)methyl]-, (+)- (9CI)](/CAS/GIF/769092-24-2.gif)