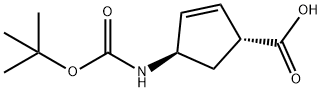
(1R,4R)-4-(tert-butoxycarbonylamino)cyclopent-2-enecarboxylic acid synthesis
- Product Name:(1R,4R)-4-(tert-butoxycarbonylamino)cyclopent-2-enecarboxylic acid
- CAS Number:298716-03-7
- Molecular formula:C11H17NO4
- Molecular Weight:227.26
![4-[[(1,1-DIMETHYLETHOXY)CARBONYL]AMINO]-2-CYCLOPENTENE-1-CARBOXYLIC ACID METHYL ESTER](/CAS/GIF/168683-02-1.gif)
168683-02-1
156 suppliers
$161.00/10g

298716-03-7
88 suppliers
inquiry
Yield:-
Reaction Conditions:
Stage #1:(1S,4R)-(-)-methyl-[[(1,1-dimethylethoxy)carbonyl]amino]cyclopent-2-ene-1-carboxylate with sodium methylate in methanol at 0; for 0.833333 h;Industry scale;
Stage #2: with lithium hydrochloride monohydrate;water in methanol;tert-butyl methyl ether at -3.5 - 10; for 22 h;Industry scale;Inert atmosphere;
Stage #3: with hydrogenchloride in methanol;tert-butyl methyl ether;water; pH=3Product distribution / selectivity;Industry scale;
Steps:
5
EXAMPLE 3: Epimerisation of (1 S,4R)-methyl 4-(te/f-butoxycarbonyl)amino cyclopent-2-enecarboxylate, 11.11 11+12 13A methanol solution of 11 (10 L, containing about 23.63 mol) is cooled to - 2°C. A solution of sodium methoxide (127.6 g, 2.36 mol) in methanol (700 mL) is added over 30 minutes, at a rate that maintains the reaction temperature at 00C or less. After 50 minutes, gas chromatography (GC) analysis indicates a product distribution of 43% compound 12, 54% compound 11 , and 3% compound 13.Acetic acid (140 mL) is added over 5 minutes, the mixture is allowed to warm to 100C, then the mass is concentrated. After 7 L of solvent has been removed, the concentrated mixture is diluted with water (2 L) and MTBE (10 L).The aqueous layer is extracted with further MTBE (1 L). The combined organic layers are concentrated to give the products 11+12+13 in a MTBE solution, with a total volume of 7.5 L.A sample (2 mL) is removed from the bulk and concentrated further, and this is found to contain 1.5 g of product and therefore 7.5 L should contain 5.625 kg, (99% mass recovery).GC analysis shows the material to be 44% compound 12, 52% compound11 , and 4% compound 13.trans Diastereoismer 12; 1H NMR (400MHz, MeOH): δ 5.91 -5.87 (1 H, m),5.85-5.81 (1 H, m), 4.74 (1 H, brs), 3.75-3.72 (1 H, m) 3.66, (3H, s), 2.60-2.49 (1 H, m), 1.89-1.78 (1 H, m), 1.46 (9H, s). cis Diastereoismer 11 ; 1H NMR (400MHz, MeOH): δ 5.91 -5.87 (1 H, m), 5.85-5.81 (1 H, m), 4.65 (1 H, brs), 3.72, (3H, s), 3.57-3.52 (1 H, m), 2.60-2.49 (1 H, m), 1.89-1.78 (1 H, m), 1.46 (9H, s). EXAMPLE 4: Ester hydrolysis.A solution of a mixture of methyl esters 11+12+13 in MTBE (3.5 L, containing about 11.0 mol) is diluted with methanol (4 L) under N2, then cooled to -3.5°C (jacket temperature -5°C). A solution of lithium hydroxide monohydrate (508 g, 12.08 mol) in water (2.5 L) is added over 5.5 hours with the reaction temperature being kept below 2°C at all times during the addition. Once the addition is complete, the mass is stirred for at 2°C for 30 minutes, then at 100C for 16 hours.The mass is neutralised with 6 M HCI and concentrated. After 4 L of solvent has been removed, the concentrated mixture is acidified to pH 3 by the addition of further 6M HCI. The acidic mixture is extracted with ethyl acetate (10 L, then 2.5 L), and the combined organics are washed with brine (2*1.5 L), then concentrated. Toluene (2*2.5 L) is added during the concentration to aid the removal of water. The products 14+15+16 are recovered as an off white solid (2.67 kg, 94% yield).trans Diastereoismer 15; 1H NMR (400MHz, MeOH): δ 5.93-5.90 (1 H, m), 5.84-5.83 (1 H, m), 4.75 (1 H, brs), 3.72-3.67 (1 H, m), 2.59-2.49 (1 H, m), 1.90-1.78 (1 H, m), 1.45 (9H, s).cis Diastereoismer 14; 1H NMR (400MHz, MeOH): δ 5.93-5.90 (1 H, m), 5.84-5.83 (1 H, m), 4.67 (1 H, brs), 3.52-3.48 (1 H, m), 2.59-2.49 (1 H, m), 1.90-1.78 (1 H, m), 1.45 (9H, s). EXAMPLE 5: Preparation of (1 R,4R)-4-(te/t-butoxycarbonyl)aminocyclopent-2- enecarboxylic acid, 15.HO2C/,.</Ny-.NHBoc14+15 16 15 A mixture of 14+15+16 (1 .81 kg, 7.99 mol) in ethyl acetate (15 L) is warmed to 500C under N2. The resulting cloudy solution is cooled to 8°C (jacket temperature 5°C) and 3-dimethylaminopropylamine (1 L, 7.99 mol) is added drop- wise over 1 .5 hours, keeping the mixture temperature below 15°C. The mixture is stirred at 25°C for 16 hours. The precipitate is collected and washed with ethyl acetate (2 L).The combined filtrates are partially concentrated and 8 L of solvent is removed. The concentrated solution is diluted with water (4 L) and acidified to pH 3 by the addition of 6 M HCI, then stirred for 30 minutes. The aqueous layer is washed with ethyl acetate (2.5 L). The combined organic layers are washed with brine (1 .5 L) and concentrated to give 15 as an off white solid (550 g, 30% yield).Liquid chromatography (LC) analysis shows the material to be 88% compound 15, 8% compound 14, and 4% compound 16.A mixture of 15, 14 and 16 in the ratio described above (1 .74 kg, 2.68 mol) is dissolved in MTBE (3.5 L) at 55°C, under N2. The solution is allowed to cool to 400C and heptane (7 L) is added. The mixture is cooled to 200C and further heptane (1 L) is added to aid stirring. The mass is stirred at 20°C for 16 hours. The solid material is collected, washed with a 1 :3 mixture of MTBE and heptane (2 L) and dried to give 15 as a white solid (1 .3 kg, 75% yield).LC analysis shows the material to be 96.4% compound 15, 2.2%compound 14, and 0.6% compound 16.This mixture (1 .30 kg, 5.73 mol) is dissolved in ethyl acetate (2.6 L) at 600C, under N2. The solution is allowed to cool to 50°C and heptane (6 L) is added. The mixture is cooled to 20°C and stirred for 16 hours. The solid material is collected, washed with a 1 :3 mixture of ethyl acetate and heptane (1 .5 L) and dried to give 15 as a white solid (1 .09 kg, 84% yield).LC analysis shows the material to be 99.4% compound 15, 0.6%compound 16.1 H NMR (400MHz, MeOH): δ 5.92-5.90 (1 H, m), 5.83-5.82 (1 H, m), 4.75 (1 H, br), 3.70-3.67 (1 H, m), 3.33-3.32 (1 H, m), 2.56-2.50 (1 H, m), 1 .88-1 .81 (1 H, m), 1 .46 (9H, s).
References:
DR. REDDY'S LABORATORIES LTD.;DR. REDDY'S LABORATORIES, INC.;LLOYD, Richard;PETERSON, Justine, Ann;JACKSON, Mark WO2011/3061, 2011, A2 Location in patent:Page/Page column 11-12
![4-[[(1,1-DIMETHYLETHOXY)CARBONYL]AMINO]-2-CYCLOPENTENE-1-CARBOXYLIC ACID METHYL ESTER](/CAS/GIF/168683-02-1.gif)
168683-02-1
156 suppliers
$161.00/10g

1312161-65-1
5 suppliers
inquiry
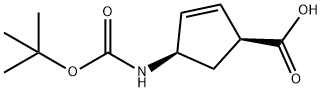
151907-79-8
162 suppliers
$20.00/100mg

298716-03-7
88 suppliers
inquiry
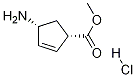
229613-83-6
40 suppliers
inquiry

298716-03-7
88 suppliers
inquiry
![((1R,4S)-2-Azabicyclo[2.2.1]hept-5-en-3-one](/CAS/GIF/79200-56-9.gif)
79200-56-9
226 suppliers
$10.00/1g

298716-03-7
88 suppliers
inquiry

229613-83-6
40 suppliers
inquiry

1312161-65-1
5 suppliers
inquiry

151907-79-8
162 suppliers
$20.00/100mg

298716-03-7
88 suppliers
inquiry