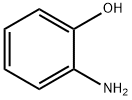
2H-1,4-Benzoxazine, 3-(4-methoxyphenyl)- synthesis
- Product Name:2H-1,4-Benzoxazine, 3-(4-methoxyphenyl)-
- CAS Number:112030-48-5
- Molecular formula:C15H13NO2
- Molecular Weight:239.27
Yield:112030-48-5 92%
Reaction Conditions:
with iodine;potassium carbonate in tetrahydrofuran;water at 20; for 7.5 h;Inert atmosphere;Green chemistry;
Steps:
General procedure for the synthesis of 1,4-benzoxazine 3:
General procedure: A flame-dried round bottom flask was charged with o-aminophenol 1 (1 equiv), 2-chloro-1-phenylethanone 2 (1 equiv), and I2 (0.1 mmol) in 5 mL of THF/H2O (5:1)under positive pressure of nitrogen followed by addition of K2CO3 (3 equiv).The resulting solution was stirred for 7-8 h at room temperature (Table 2).After completion of the reaction (monitored by TLC), the reaction mixture wasquenched with satd aq Na2SO3 until the iodine color almost disappeared and was extracted with CHCl3. The organic layer was washed with satd aq NaHCO3and brine, and dried over Na2SO4. Then the combined organic layer was concentrated under reduced pressure. The residue was purified by flash column chromatography on silica gel using hexane/EtOAc (10:1) as eluent toafford analytically pure sample of 3. The structure of products was confirmed by comparison of their mp, TLC, IR, and 1H, 13C NMR data with authentic samples obtained commercially or prepared by the literature methods.6,12 Physical data of representative compound. Compound 3c: yellow solid, mp130-132 C, 92% yield. 1H NMR (400 MHz, CDCl3) δ: 3.94 (s, 3H), 5.01 (s, 2H),6.92 (d, J = 8.0 Hz, 1H), 7.09-7.15 (m, 2H), 7.19 (t, J = 5.9 Hz, 1H), 7.32-7.40 (m,3H), 7.53 (s, 1H). 13C NMR (100 MHz, CDCl3) δ: 55.4, 73.9, 113.2, 115.4, 116.9,122.1, 123.5, 124.7, 127.9, 128.9, 134.7, 139.8, 153.2, 161.4, 164.7. EIMS (m/z):239 (M+). Anal. Calcd for C15H13NO2: C, 75.30; H, 5.48; N, 6.27. Found: C, 75.00;H, 5.80; N, 5.56.
References:
Singh, Sunil K.;Bajpai, Anil K.;Saini, Rajesh [Tetrahedron Letters,2013,vol. 54,# 52,p. 7132 - 7135]
74413-02-8
0 suppliers
inquiry

112030-48-5
1 suppliers
inquiry
100-06-1
583 suppliers
$18.19/25g

112030-48-5
1 suppliers
inquiry
2632-13-5
357 suppliers
$5.00/25mg

112030-48-5
1 suppliers
inquiry