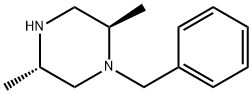
(2R,5S)-1-benzyl-2,5-dimethylpiperazine synthesis
- Product Name:(2R,5S)-1-benzyl-2,5-dimethylpiperazine
- CAS Number:216532-43-3
- Molecular formula:C13H20N2
- Molecular Weight:204.31
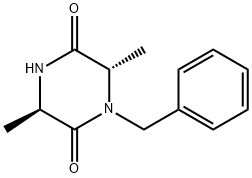
1072102-01-2
0 suppliers
inquiry

216532-43-3
32 suppliers
inquiry
Yield:216532-43-3 99%
Reaction Conditions:
with lithium aluminium tetrahydride in tetrahydrofuran at 80; for 5.66667 h;Cooling with ice;Inert atmosphere;
Steps:
14.1.a.2.1.4
a-2-1-4) Preparation of (2R,5S)-1-benzyl-2,5-dimethylpiperazine To a solution of (3R,6S)-1-benzyl-3,6-dimethylpiperazine-2,5-dione (4.05 g, 17.4 mmol) in tetrahydrofuran (70 mL), added lithium aluminum hydride (993 mg, 26.2 mmol) under ice-cold conditions, under an argon atmosphere. The mixture was stirred at room temperature for 10 minutes, and then stirred at 80° C. for 4 hours. Then, the reaction solution was added lithium aluminum hydride (331 mg, 8.72 mmol) under ice-cold conditions and stirred at 80° C. for 1.5 hours. Then, the reaction solution was added tetrahydrofuran and water under ice-cold conditions, and stirred at room temperature overnight. The reaction solution was filtered through a pad of celite, washed with ethyl acetate and water, dried over sodium sulfate, then concentrated in vacuo, and the title compound (3.52 g (yield 99%)) was obtained as a yellow oil. 1H-NMR (CDCl3) δ: 0.93 (3H, d, J=6.3 Hz), 1.14 (3H, d, J=6.1 Hz), 1.63 (1H, dd, J=10.2, 11.2 Hz), 2.18-2.26 (1H, m), 2.63 (1H, dd, J=10.5, 12.2 Hz), 2.68 (1H, dd, J=2.7, 11.2 Hz), 2.75-2.83 (1H, m), 2.91 (1H, dd, J=2.9, 12.2 Hz), 3.09 (1H, d, J=13.4 Hz), 4.10 (1H, d, J=13.4 Hz), 7.22-7.32 (5H, m).
References:
US2010/280013,2010,A1 Location in patent:Page/Page column 28
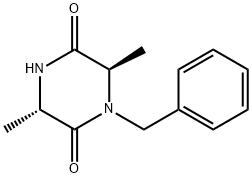
262295-89-6
0 suppliers
inquiry

216532-43-3
32 suppliers
inquiry

100-39-0
423 suppliers
$10.00/10g
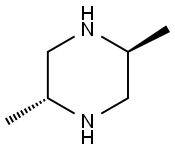
1119702-25-8
3 suppliers
inquiry

260254-80-6
32 suppliers
inquiry

216532-43-3
32 suppliers
inquiry

1119702-25-8
3 suppliers
inquiry

216532-43-3
32 suppliers
inquiry

100-44-7
625 suppliers
$13.50/250G

1119702-25-8
3 suppliers
inquiry

216532-43-3
32 suppliers
inquiry