
(2S,3R)-2,3-Hexanediol synthesis
- Product Name:(2S,3R)-2,3-Hexanediol
- CAS Number:22520-19-0
- Molecular formula:C6H14O2
- Molecular Weight:118.17
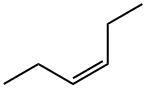
7642-09-3
45 suppliers
$18.00/1mL

22520-19-0
0 suppliers
inquiry
Yield:22520-19-0 77%
Reaction Conditions:
with 4-methylmorpholine N-oxide in water;acetone at 100; for 3 h;
Steps:
General procedure for the dihydroxylation of alkenes
General procedure: alkenesTo a stirred solution of alkene (1, 1 mmol) in a mixture ofacetone:H2O 2:1 (3 mL) in a pressure tube, OsO2-Fe3O4(10 mg,0.08% of osmium) and NMO (234 mg, 2 mmol) were added. Theresulting mixture was stirred at 100C during 3 h. The catalyst wasremoved by a magnet and the resulting solution was extracted withether. The organic phases were dried over MgSO4, and the solventswere removed under reduced pressure. The product was usuallypurified by chromatography on silica gel (hexane/ethyl acetate)to give the corresponding products 2 or 4. Physical and spectro-scopic data as well as literature for all compounds are includedas Appendices A and B. FT-IR spectra were obtained on a Nicoletimpact 400D spectrophotometer. NMR spectra were recorded ona Bruker AC-300 apparatus (300 MHz for1H and 75 MHz for13C)using CDCl3as a solvent and TMS as internal standard for1H and13C; chemical shifts are given in (parts per million) and couplingconstants (J) in Hertz. Mass spectra (EI) were obtained at 70 eVon a spectrometer Agilent GC/MS-5973N, giving fragment ions inm/z with relative intensities (%) in parentheses. Thin layer chro-matography (TLC) was carried out on DC-Fertigfolien ALUGRAMplates coated with a 0.2 mm layer of silica gel; detection by UV254light, staining with phosphomolybdic acid [25 g phosphomolybdicacid, 10 g Ce(SO4)2·4H2O, 60 mL of concentrated H2SO4and 940 mLH2O]. Column chromatography was performed using silica gel 60of 35-70 mesh.
References:
Cano, Rafael;Pérez, Juana M.;Ramón, Diego J. [Applied Catalysis A: General,2014,vol. 470,p. 177 - 182]

13269-52-8
61 suppliers
$31.30/1g

22520-19-0
0 suppliers
inquiry
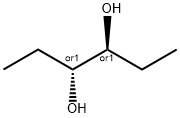
22520-39-4
0 suppliers
inquiry

22520-19-0
0 suppliers
inquiry
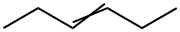
592-47-2
37 suppliers
$162.00/5mL

22520-19-0
0 suppliers
inquiry

