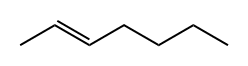
TRANS-3-HEXENE synthesis
- Product Name:TRANS-3-HEXENE
- CAS Number:13269-52-8
- Molecular formula:C6H12
- Molecular Weight:84.16
Yield: 51.9 %Spectr. , 45.2 %Spectr.
Reaction Conditions:
with C21H34N3PRu(1+)*F6P(1-) in [(2)H6]acetone at 20; for 4 h;Glovebox;Inert atmosphere;Schlenk technique;Time;
Steps:
I.VI-2a
tris(acetonitrile)Pentamethylcyclopentadienylruthenium(II) hexafluorophosphate (116.9 mg, 0.2317 mmol) was weighed in a scintillation vial equipped with a magnetic stir bar in a glove box, and deoxygenated acetone (6 mL) was added. In a separate vial, the requisite phosphine (59.4 mg, 0.2336 mmol) was weighed and deoxygenated acetone (1 mL) was added. The phosphine dissolved in acetone was added dropwise to the ruthenium complex solution, and deoxygenated acetone was used to rinse the vial. The mixture was allowed to stir in the glovebox at ambient temperature overnight, though formation of 3 occurs within 15 min [see below]. The solvent was evaporated forming a foam, to which was added acetone. The process was repeated four times within 2 h. After concentration, the residue was stored under vacuum to afford a yellow-brown foam (152.6 mg vs. 156.7 mg theoretical yield for pure 1 and 166.3 mg for pure 3; given composition, yield is about 93%). Following the general procedure, 1 -heptene (49.4 mg,0.503 mmol) and catalyst 2a (3.0 mg, 0.0050 mmol, 1 mol%) were used. Reaction was conducted at ambient temperature. At specified time points, ‘H NMR spectra wereobtained.
References:
San Diego State University Research Foundation;Grotjahn, Douglas;Larsen, Casey;Erdogan, Gulin;Paulson, Erik US9708236, 2017, B2 Location in patent:Page/Page column 49-52
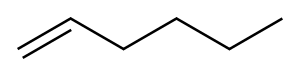
592-41-6
251 suppliers
$10.00/5g

13269-52-8
61 suppliers
$31.30/1g
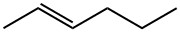
4050-45-7
60 suppliers
$33.50/1 g

592-41-6
251 suppliers
$10.00/5g

13269-52-8
61 suppliers
$31.30/1g
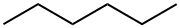
110-54-3
778 suppliers
$20.00/100ml
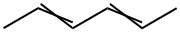
592-46-1
52 suppliers
$28.50/1g

592-41-6
251 suppliers
$10.00/5g
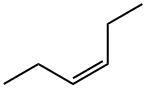
7642-09-3
45 suppliers
$20.00/1mL

13269-52-8
61 suppliers
$31.30/1g
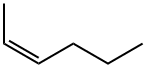
7688-21-3
52 suppliers
$32.70/1g
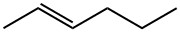
4050-45-7
60 suppliers
$33.50/1 g

928-49-4
76 suppliers
$24.00/5mL

7642-09-3
45 suppliers
$20.00/1mL

13269-52-8
61 suppliers
$31.30/1g

7688-21-3
52 suppliers
$32.70/1g
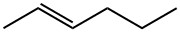
4050-45-7
60 suppliers
$33.50/1 g