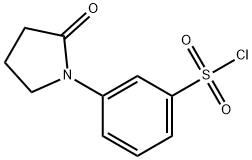
3-(2-O×o-pyrrolidin-1-yl)benzenesulfonyl chloride synthesis
- Product Name:3-(2-O×o-pyrrolidin-1-yl)benzenesulfonyl chloride
- CAS Number:344312-54-5
- Molecular formula:C10H10ClNO3S
- Molecular Weight:259.71
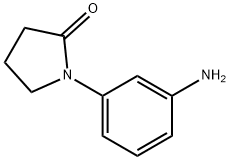
31992-43-5
37 suppliers
$42.00/500mg

344312-54-5
10 suppliers
inquiry
Yield:344312-54-5 22%
Reaction Conditions:
Stage #1: 1-(3-aminophenyl)pyrrolidin-2-onewith hydrogenchloride;acetic acid;sodium nitrite in water;acetonitrile at 0; for 1 h;
Stage #2: with sulfur dioxide in water;acetonitrile at 0; for 2 h;
Stage #3: with sulfur dioxide;copper dichloride in water;acetonitrile at 0 - 20; for 17 h;
Steps:
5.3
Hydrochloric acid (11 mL) was added to a solution of l-(3-aminophenyl)pyrrolidin-2-one (35.2 mmol) in acetic acid (21 mL) and acetonitrile (250 mL)at 0 °C. A solution of sodium nitrite (42.0 mmol) in water (3 mL) was subsequently added and the mixture was maintained for 60 min at 0 °C. Sulfur dioxide gas was bubbled through the solution for 2 h while the temperature was maintained at 0 oC. A solution of copper(II) chloride dihydrate (38.8 mmol) in water (5 mL) was added dropwise and sulfur
References:
WO2009/23844,2009,A2 Location in patent:Page/Page column 92-93
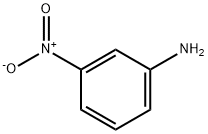
99-09-2
10 suppliers
$14.00/1g

344312-54-5
10 suppliers
inquiry
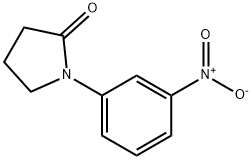
61372-79-0
22 suppliers
$28.60/50MG

344312-54-5
10 suppliers
inquiry