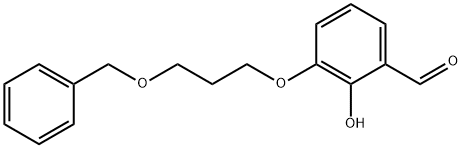
3-(3-(benzyloxy)propoxy)-2-hydroxybenzaldehyde synthesis
- Product Name:3-(3-(benzyloxy)propoxy)-2-hydroxybenzaldehyde
- CAS Number:1093644-47-3
- Molecular formula:C17H18O4
- Molecular Weight:286.32
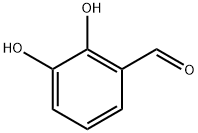
24677-78-9
253 suppliers
$8.00/1g
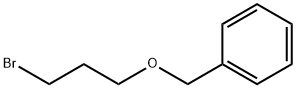
54314-84-0
221 suppliers
$8.00/1g

1093644-47-3
6 suppliers
inquiry
Yield:1093644-47-3 84.31%
Reaction Conditions:
Stage #1: 2,3-dihydroxybenzaldehydewith sodium t-butanolate in dimethyl sulfoxide at 20; for 0.5 h;
Stage #2: 1-[(3-bromopropoxy)methyl]-benzene in dimethyl sulfoxide at 0 - 20;Product distribution / selectivity;
Steps:
1
3-(3-Benzyloxy-propoxy)-2-hvdroxy-benzaldehvde (4)[0717] To a solution of aldehyde 3 (27.47 g, 1 eq, 198.88 mmol) in 0.5 L of anhydrous DMSO was added sodium tertiary-butoxide (42.3 g, 2.2 eq, 440.31 mmol) portionwise. The reaction mixture was stirred at rt for 30 minutes. A brown color solution was formed. The reaction mixture was cooled to O0C and added bromide (56 g, 1.2 eq, 244.41 mmol) dropwise. The mixture was stirred at rt O/N. 90% of aldehyde 3 was converted to product. The reaction mixture was acidified to pH~3 and then extracted into EtOAc and washed with water. The organic layer was concentrated, the product was purified on silica gel column (EtOAc:hexane 80:20), to yield as compound 4 (48 g, 84.31 % yield) (viscous oil).
References:
WO2008/157726,2008,A1 Location in patent:Page/Page column 220

4799-68-2
178 suppliers
$5.00/1g

1093644-47-3
6 suppliers
inquiry