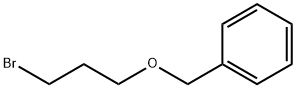
BENZYL 3-BROMOPROPYL ETHER synthesis
- Product Name:BENZYL 3-BROMOPROPYL ETHER
- CAS Number:54314-84-0
- Molecular formula:C10H13BrO
- Molecular Weight:229.11

4799-68-2
177 suppliers
$5.00/1g

54314-84-0
221 suppliers
$8.00/1g
Yield:54314-84-0 99%
Reaction Conditions:
with carbon tetrabromide;Cu(tmp)(BINAP)BF4;sodium bromide in N,N-dimethyl-formamide for 24 h;Catalytic behavior;UV-irradiation;Inert atmosphere;Appel Halogenation;Reagent/catalyst;Time;
Steps:
General procedure for the Appel reaction in batch
General procedure: To an open oven-dried reaction vial charged with a stir bar was added copper catalyst (2 mg,0.002 mmol, 0.01 equiv), the alcohol (0.20 mmol, 1.0 equiv), carbon tetrabromide (131.6 mg, 0.4mmol, 2.0 equiv) and sodium bromide (41 mg, 0.40 mmol, 2.0 equiv). The flask vial was capped,purged with a stream of nitrogen and dry DMF (1.5 mL) was added via syringe. The reactionmixture was stirred under purple LEDs (394 nm) for 24 h. The vessel was opened and the mixturewas poured into a separatory funnel containing Et2O (10 mL) and H2O (10 mL). The layers wereseparated, and the aqueous layer was extracted with Et2O (2 × 10 mL). The combined organiclayers were washed with sat. Na2S2O3 solution, brine, dried over Na2SO4 and concentrated invacuo. The residue was purified by chromatography (100 % hexanes)
References:
Minozzi, Clémentine;Grenier-Petel, Jean-Christophe;Parisien-Collette, Shawn;Collins, Shawn K. [Beilstein Journal of Organic Chemistry,2018,vol. 14,p. 2730 - 2736] Location in patent:supporting information
14593-43-2
119 suppliers
$18.00/1g

54314-84-0
221 suppliers
$8.00/1g
100-39-0
425 suppliers
$10.00/10 g

627-18-9
372 suppliers
$9.00/1g

54314-84-0
221 suppliers
$8.00/1g