
3,3-DIBROMO-5-CHLORO-1,3-DIHYDROINDOL-2-ONE synthesis
- Product Name:3,3-DIBROMO-5-CHLORO-1,3-DIHYDROINDOL-2-ONE
- CAS Number:113423-48-6
- Molecular formula:C8H4Br2ClNO
- Molecular Weight:325.38
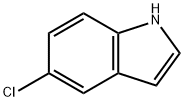
17422-32-1
369 suppliers
$6.00/1g

113423-48-6
5 suppliers
inquiry
Yield:-
Reaction Conditions:
with pyridinium hydrobromide perbromide in tert-butyl alcohol;
Steps:
1 3,3-Dibromo-5-chloroindol-2(3H)-one
EXAMPLE 1 3,3-Dibromo-5-chloroindol-2(3H)-one To a solution of 5-chloroindole (1.00 g) in t-butanol (65 ml) was added portionwise over 0.5 hr 6.9 g of pyridinium bromide perbromide. The reaction mixture was stirred at room temperature for 2 hours after which TLC analysis (1:1 ethyl acetate/hexane) indicated complete conversion of starting material to several products. The reaction mixture was diluted with ethyl acetate (400 ml) and H2 O (400 ml). The organic layer was separated and the aqueous layer extracted with ethyl acetate (300 ml). The combined organic extracts were washed with H2 O (2*400 ml) and brine, dried (Na2 SO4) and concentrated in vacuo to a yellow-green oil. Purification on a silica gel column eluted with 40% ethyl acetate/hexane afforded the more polar product (3,3-dibromo-5-chlorooxindole) as a brown solid (1.26 g, 60%).
References:
US4761485,1988,A
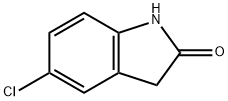
17630-75-0
284 suppliers
$10.00/1g

113423-48-6
5 suppliers
inquiry