
3,3-Difluorocyclobutylmethyl tosylate synthesis
- Product Name:3,3-Difluorocyclobutylmethyl tosylate
- CAS Number:681128-40-5
- Molecular formula:C12H14F2O3S
- Molecular Weight:276.3
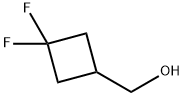
681128-39-2
138 suppliers
$25.00/250mg

98-59-9
601 suppliers
$9.00/5g

681128-40-5
19 suppliers
inquiry
Yield:681128-40-5 98%
Reaction Conditions:
with pyridine in dichloromethane at 0 - 20; for 18 h;
Steps:
316A (3,3-Difluorocyclobutyl)methyl 4-methylbenzenesulfonate
To a solution of 4.0 g (31.1 mmol, 95% purity) of (3,3-difluorocyclobutyl)methanol in 60 ml of dichloromethane were added 7.6 ml (93.4 mmol) of pyridine, and the mixture was cooled to 0° C.
At this temperature, 6.53 g (34.2 mmol) of para-toluenesulfonyl chloride were added in portions.
Then the reaction mixture was stirred at RT for about 18 h.
Subsequently, it was diluted with dichloromethane, washed with water, dried over anhydrous magnesium sulfate, filtered and concentrated. 8.65 g (98% of theory, 97% purity) of the title compound were obtained.
1H-NMR (400 MHz, DMSO-d6, δ/ppm): 7.80 (d, 2H), 7.50 (d, 2H), 4.09 (d, 2H), 2.67-2.56 (m, 2H), 2.43 (s, 3H), 2.37-2.21 (m, 3H).
GC/MS (Method 9, EI): Rt=6.13 min, m/z=276 [M]+.
References:
US2020/16159,2020,A1 Location in patent:Paragraph 1592-1595
107496-54-8
214 suppliers
$29.00/1g

681128-40-5
19 suppliers
inquiry