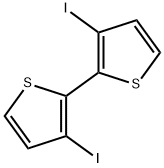
3,3'-diiodo-2,2'-bithiophene synthesis
- Product Name:3,3'-diiodo-2,2'-bithiophene
- CAS Number:90348-22-4
- Molecular formula:C8H4I2S2
- Molecular Weight:418.06
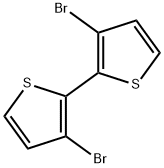
51751-44-1
234 suppliers
$7.00/100mg

90348-22-4
2 suppliers
inquiry
Yield:90348-22-4 65%
Reaction Conditions:
Stage #1: 3,3'dibromo-2,2'-bithiophenewith n-butyllithium in diethyl ether;hexane at -78; for 1 h;
Stage #2: with iodine in diethyl ether;hexane at 20; for 1 h;
Steps:
1
(Synthesis Example 1: Synthesis of 3,3'-diiodo-2,2'-bithiophene); First, the starting raw material 3,3'-dibromo-2,2'-bithiophene was synthesized by referring to the description in a reference document (Hong M., Wei H., J. Org. Chem., 2000, 65, 3895). Then, a halogen exchange reaction was carried out using this starting raw material to synthesize 3,3'-diiodo-2,2'-bithiophene. Specifically, first, a three-necked 300 mL flask was charged with 3,3'-dibromo-2,2'-bithiophene (2.7 g (7 mmol)), which was then dissolved in diethyl ether (70 mL). Next, the contents of the reaction vessel were purged with nitrogen and the vessel was cooled to -78°C. Then, the vessel was charged with butyllithium (in 1.5 M hexane solution, 10.3 mL (15.4 mmol)), and the resultant solution was stirred for 1 hour. Iodine (3.9 g (15.4 mmol) dissolved in diethyl ether was further charged thereto, and the resultant solution was reacted by stirring for 1 hour at room temperature. The post-reaction solution was charged with diethyl ether (about 50 mL), and the resultant solution was then washed with a saturated aqueous sodium thiosulfate solution. Subsequently, the organic layer was dried over sodium sulfate, and then filtered over Celite. The solvent was removed from the filtrate by distillation, and then the resultant solid was recrystallized with hexane and toluene to obtain the target product 3,3'-diiodo-2,2'-bithiophene as a white solid (1.9 g, 65% yield). The melting point of the obtained white solid was measured to be 148°C. (Document value 149.5 to 151°C; Gronowitz S., Vilks V., Arkiv Kemi, 1963, 21, 191.)
References:
EP2006291,2008,A1 Location in patent:Page/Page column 17
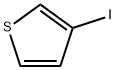
10486-61-0
159 suppliers
$6.00/1g

90348-22-4
2 suppliers
inquiry
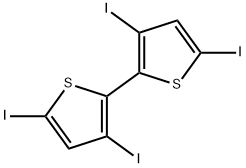
90007-99-1
0 suppliers
inquiry

90348-22-4
2 suppliers
inquiry