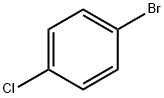
3-(4-CHLORO-PHENYL)-PYRIDINE synthesis
- Product Name:3-(4-CHLORO-PHENYL)-PYRIDINE
- CAS Number:5957-97-1
- Molecular formula:C11H8ClN
- Molecular Weight:189.64
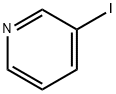
626-55-1
539 suppliers
$5.00/5/ G
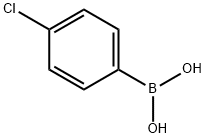
1679-18-1
589 suppliers
$10.00/1g

5957-97-1
22 suppliers
$355.00/1g
Yield:5957-97-1 100%
Reaction Conditions:
with tetrakis-(triphenylphosphine)-palladium;potassium carbonate in ethanol;lithium hydroxide monohydrate;toluene at 75; for 24 h;Inert atmosphere;Reflux;
Steps:
2.I
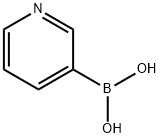
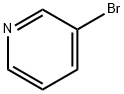
In other words, under nitrogen, to an 1L flask equipped witha stirrer& cooling tube, 3-Promo pyridine 11.5g (72.7mmol), 4- chlororphenyl boronacid 12.5g (79.9mmol), toluene 200mL, ethanol 100 mL and 2M aqueous solution ofpotassium carbonate 145mL were put and nitrogen bubbling was done for 7 minuteswhile stirring.There to, Pd (PPh3)4, 842mg (0.727mmol) was added and themixture was refluxed for 24 hours at a liquid temperature of 75 . After cooling, the separated lowermost layer in 3 layers was removedby liquid separation, the upper layer was washed twice with 5% brine 140mL by liquid separation. The resulting organic layer was dried with sodiumsulfate, and sodium sulfate was removed by filtration and evaporated. To givean oil 16.2g of turret yellow color. The resulting oil was purified by silicagel column chromatography (silica gel 200 g, ethyl acetate / hexane = 1/1) togive the compound 1 of 14.2 g. The yield was 100%. Compound 1 was a yellow oil.
References:
JP2015/214512,2015,A Location in patent:Paragraph 0034-0036
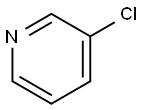
626-60-8
358 suppliers
$13.00/5g
873-77-8
145 suppliers
$65.29/100ml

5957-97-1
22 suppliers
$355.00/1g

59-67-6
1102 suppliers
$7.00/25g

1679-18-1
589 suppliers
$10.00/1g

5957-97-1
22 suppliers
$355.00/1g