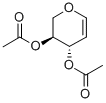
3,4-DI-O-ACETYL-L-ARABINAL synthesis
- Product Name:3,4-DI-O-ACETYL-L-ARABINAL
- CAS Number:3945-18-4
- Molecular formula:C9H12O5
- Molecular Weight:200.19
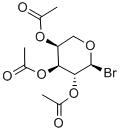
75247-31-3
12 suppliers
inquiry

3945-18-4
16 suppliers
$60.00/500mg
Yield:3945-18-4 91%
Reaction Conditions:
with ammonium chloride;zinc;bis(acetylacetonate)oxovanadium in methanol at 20; for 0.166667 h;Product distribution / selectivity;
Steps:
3
L-arabinose is converted to the corresponding glycal derivative via a key reductive elimination step and converting the resulting glycal intermediate to methyl 2-deoxy ribofuranoside. See M. L. Sznaidman, M. R. Almond, and A. Pesyan. Nucleosides, Nucleotides & Nucleic Acids 2002, 21, 155-163; Z.-X. Wang, W. Duan, L. I. Wiebe, J. Balzarini, E. D. Clercq, and E. E. Knaus, Nucleosides, Nucleotides & Nucleic Acids 2001, 20, 11-40; and R. V. Stick, K. A. Stubbs, D. M. G. Tilbrook, and A. G. Watts, Aust. J. Chem. 2002, 55, 83-85.
References:
US2005/59632,2005,A1 Location in patent:Page/Page column 21
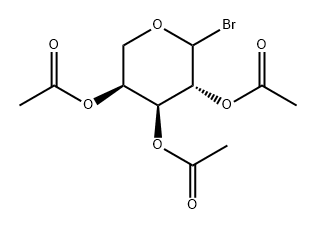
51830-02-5
1 suppliers
inquiry

3945-18-4
16 suppliers
$60.00/500mg

14227-90-8
20 suppliers
inquiry

3945-18-4
16 suppliers
$60.00/500mg
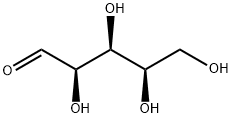
58-86-6
422 suppliers
$5.00/25g

108-24-7
5 suppliers
$14.00/250ML

3945-18-4
16 suppliers
$60.00/500mg
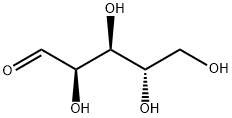
5328-37-0
460 suppliers
$5.00/1g

108-24-7
5 suppliers
$14.00/250ML

3945-18-4
16 suppliers
$60.00/500mg