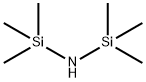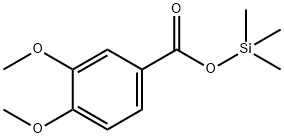
3,4-Dimethoxybenzoic acid trimethylsilyl ester synthesis
- Product Name:3,4-Dimethoxybenzoic acid trimethylsilyl ester
- CAS Number:2078-16-2
- Molecular formula:C12H18O4Si
- Molecular Weight:254.35
Yield:2078-16-2 94%
Reaction Conditions:
in neat (no solvent) at 20; for 40 h;Green chemistry;
Steps:
22 4.2. Representative procedure of the non-catalyzed functionalization of carboxylic acids with HMDS under SFC
General procedure: To octanoic acid 1a (4.32 g, 30 mmol) HMDS (3.63 g, 22.5 mmol) was added, and the mixture was stirred at room temperature until the complete consumption 1a. The initial heterogeneous reaction mixture became homogeneous, and 1H NMR analysis confirmed full conversion. The excess of HMDS was distilled off under reduced pressure, and distillation of the crude product furnished trimethylsilyl octanoate 2a (5.91 g, 91%) as a transparent oil. The distilled excess of HMDS could be reused.
References:
Jereb, Marjan;Lakner, Janja [Tetrahedron,2016,vol. 72,# 37,p. 5713 - 5723]
93-03-8
376 suppliers
$5.00/1g

2078-16-2
0 suppliers
inquiry
1131-62-0
316 suppliers
$14.00/5g

2078-16-2
0 suppliers
inquiry
![3,4-dimethoxy-α-[(2-methoxyphenoxy)methyl]-Benzenemethanol](/CAS/20181212/GIF/17078-88-5.gif)
17078-88-5
1 suppliers
inquiry

2078-16-2
0 suppliers
inquiry

22675-96-3
0 suppliers
inquiry

2078-16-2
0 suppliers
inquiry