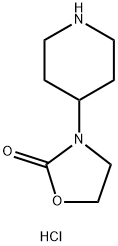
3-(4-Piperidinyl)-2-oxazolidinone HCl synthesis
- Product Name:3-(4-Piperidinyl)-2-oxazolidinone HCl
- CAS Number:130818-98-3
- Molecular formula:C8H15ClN2O2
- Molecular Weight:206.67
![2-Oxazolidinone, 3-[1-(phenylmethyl)-4-piperidinyl]-](/CAS/20210305/GIF/130818-97-2.gif)
130818-97-2
0 suppliers
inquiry

130818-98-3
20 suppliers
$90.00/50mg
Yield:130818-98-3 99%
Reaction Conditions:
Stage #1: 3-(1-benzylpiperidin-4-yl)oxazolidin-2-onewith 4-Chlorobutanoyl chloride in 1,2-dichloro-ethane at 100; for 1.5 h;
Stage #2: in methanol; for 1 h;Heating / reflux;
Steps:
259.A
EXAMPLE 259; 3- {1- [4- (BENZOTHIAZOL-2-YLOXY)-BENZYL]-PIPERIDIN-4-YL}-OXAZOLIDIN-2-ONE; A. 3-Piperidin-4-yl-oxazolidin-2-one hydrochloride salt; To a solution of 1- BENZYL-4-PIPERIDINONE (10.3 g, 54 MMOL) and ETHANOLAMINE (13.2 ML, 218 MMOL) in CH30H (20 mL) was added sodium CYANOBOROHYDRIDE (10.2 g, 163 MMOL) and TRIFLUOROMETHANESULFONIC acid (5 mL) and the reaction was stirred at 23 °C for 3 days. The mixture was cooled to 0 °C and 12 N HCI was slowly added until gas evolution ceased and the resulting mixture was stirred for a further 3 h. The mixture was filtered and the filtrate concentrated under reduced pressure. The crude oil was REDISSOLVED in H20 (50 mL), the solution was made basic by the addition of 10 N NaOH. The mixture was extracted with CH2CI2 (8 x 70 mL). The combined CH2CI2 extracts were dried and concentrated under reduced pressure to yield the crude product (12.0 g, 95%). A solution OF 2- (1-BENZYL-PIPERIDIN-4-YLAMINO)-ETHANOL (3.6 g, 15.3 MMOL) in CICH2CH2CI (5 mL) was treated with carbonyl DIIMIDAZOLE (CDI) (2.6 g, 16 MMOL) and the mixture stirred at 23 °C for 30 min. The mixture was diluted with CH2CI2 (100 mL), washed with H20 (1 x 50 mL) and sat. aq. NaHCO3 (1 x 50 mL), dried, and concentrated under reduced pressure to yield 2.85 g (65%) of 3- (1-BENZYL-PIPERIDIN-4-YL)-OXAZOLIDIN-2-ONE. To a solution of 3- (1-benzyl- piperidin-4-yl)-oxazolidin-2-one (2.3 g, 8.8 MMOL) in CICH2CH2CI (40 mL) was added A-CHLOROETHYL acetylchloride (1.5 g, 10.6 MMOL) and the mixture was heated to 100 °C for 90 min. The mixture was cooled to 23 °C and concentrated under reduced pressure. The crude residue was dissolved in CH30H and heated to reflux for 1 h. The mixture was cooled to 0 °C and concentrated under reduced pressure to yield the title compound (1.89 g, 99%). MS (ESI) : exact mass calculated for C8HQ4N202, 170.1 ; m/z found, 171.2 [M+H] +. 1H NMR (400 MHz, DMSO-D6) : 8.97 (br s, 2H), 4.27 (dd, J= 9.1, 7.8, 2H), 3.80 (tt, J = 11.8, 4.2, 1 H), 3.49 (dd, J = 8.0, 6.6, 1 H), 3.30 (BR D, J= 12.7, 2H), 2.97 (dt, J= 12.6, 2.3, 2H), 1.90 (ddd, J= 16.6, 13.0, 4. 1,/2H), 1.86-1. 75 (m, 2H)
References:
WO2005/12296,2005,A1 Location in patent:Page/Page column 175-176
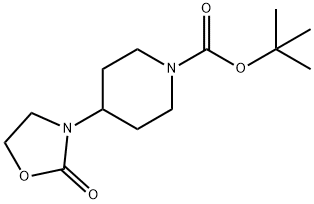
1148003-95-5
1 suppliers
inquiry

130818-98-3
20 suppliers
$90.00/50mg
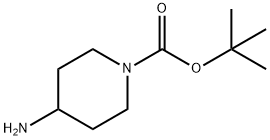
87120-72-7
20 suppliers
$6.00/1g

130818-98-3
20 suppliers
$90.00/50mg