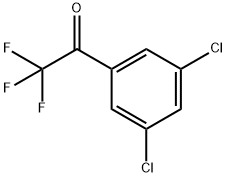
3',5'-DICHLORO-2,2,2-TRIFLUOROACETOPHENONE synthesis
- Product Name:3',5'-DICHLORO-2,2,2-TRIFLUOROACETOPHENONE
- CAS Number:130336-16-2
- Molecular formula:C8H3Cl2F3O
- Molecular Weight:243.01

431-47-0
294 suppliers
$10.00/5g
19752-55-7
371 suppliers
$8.00/10g

130336-16-2
338 suppliers
$7.00/100mg
Yield:130336-16-2 75.7%
Reaction Conditions:
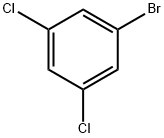
Stage #1:1-bromo-3,5-dichlorobenzene with TurboGrignard in tetrahydrofuran at 20 - 25; for 1.75 h;
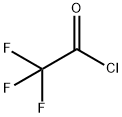
Stage #2:trifluoroacetic acid-methyl ester in tetrahydrofuran at -10 - 20; for 0.2 h;
Stage #3: with hydrogenchloride in tetrahydrofuran;water at -10 - 0; for 0.5 h;
Steps:
3 1-(3,5-dichlorophenyl)-2,2,2-trifluoroethanone
To a solution of 1-bromo-3,5-dichlorobenzene (25.0 g, 1 10.6 mmol) in 400 ml THF at room temperature, isopropyl magnesium chloride lithium chloride complex (85.0 ml, 1.3M THF, 1 10.6 mmol) was added dropwise at room temperature over a period of 15 minutes while holding the reaction temperature between 20°C and 25°C. After the addition was complete, the reaction was allowed to stir 1.5 hours at room temperature. The reaction solution was then cooled to -5 °C to -10 °C with ice/MeOH. Methyltrifluoroacetate (12.23 ml, 121 .6 mmol) in 20 ml THF was added dropwise to the reaction solution while maintaining the reaction temp below 0 °C (~ 30 min). The reaction was stirred at -5 °C for 0.5 hr then was allowed to warm to room temperature and stir for 1.5 hrs. The reaction was cooled again to -5 °C to -10 °C then 73.7 ml 6M HCI diluted to 150 ml total volume water was added dropwise while keeping the temperature below 0 °C. Once the addition was complete, the reaction was stirred 0.5 hr at 0 °C then was allowed to warm to room temperature. Excess water was added and the resulting organic layer that separated was drawn off. The aqueous layer was washed repeatedly with DCM. The combined DCM washes and recovered organic layer were dried over sodium sulfate and concentrated to yield 90 g of an oil. The crude material was then passed through a silica gel plug (neat heptane to neat DCM) and purified by vacuum distillation (3 torr, product fractions recovered froml 12 °C to 125 °C) to provide 20.3 g product (75.7% yield). 1 H NMR (400 MHz, CHLOROFORM-d) δ ppm 7.71 (s, 1 H) 7.93 (s, 2 H). IR 1728.1 cm"1
References:
AVISTA PHARMA SOLUTIONS, INC.;SPEAKE, Jason, D. WO2018/9751, 2018, A1 Location in patent:Page/Page column 44-45

19752-55-7
371 suppliers
$8.00/10g
407-25-0
464 suppliers
$9.00/1g

130336-16-2
338 suppliers
$7.00/100mg
354-32-5
124 suppliers
$55.00/10g

19752-55-7
371 suppliers
$8.00/10g

130336-16-2
338 suppliers
$7.00/100mg
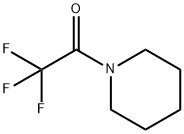
340-07-8
73 suppliers
$6.00/1g

19752-55-7
371 suppliers
$8.00/10g

130336-16-2
338 suppliers
$7.00/100mg
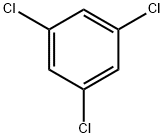
108-70-3
254 suppliers
$19.89/442235
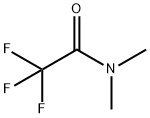
1547-87-1
137 suppliers
$6.00/1g

130336-16-2
338 suppliers
$7.00/100mg