
3,5-Dichlorofluorobenzene synthesis
- Product Name:3,5-Dichlorofluorobenzene
- CAS Number:1435-46-7
- Molecular formula:C6H3Cl2F
- Molecular Weight:164.99
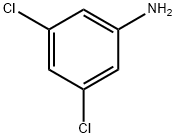
626-43-7
316 suppliers
$10.00/1g

1435-46-7
131 suppliers
$8.00/1g
Yield: 87%
Reaction Conditions:
Stage #1:3,5-Dichloroaniline with hydrogenchloride;fluoroboric acid;sodium nitrite in water at 25; for 0.00555556 h;Balz-Schiemann Reaction;
Stage #2: in 1,2-dichloro-benzene at 160; for 0.0166667 h;Balz-Schiemann Reaction;
Steps:
Typical continuous fluorodediazoniation procedure:
General procedure: 14 Typical continuous diazotization procedure: Material A (50 mL of aqueous solution containing amine (100 mmol), fluoroboric acid (120 mmol), hydrochloric acid (180 mmol)), and material B (50 mL of aqueous solution containing sodium nitrite (105 mmol)) were pumped into the T-joint at 4 mL/min, respectively, after a residence time of about 15 s at 25 °C in a reacting tube, the mixture flowed through the outlet and accumulated in the cooling vessel. Vigorous stirring was maintained. The solid was filtered with suction after the slurry was cooled to -5 °C. The solid was washed with methanol and then dried in vacuo to yield the corresponding diazonium tetrafluoroborate. 15 Typical continuous fluorodediazoniation procedure: Slurry of the diazonium tetrafluoroborate prepared as above in 300 mL of cosolvent was introduced into a reacting tube continuously at a flow rate of 4 mL/min. The mixture was maintained for 1 min at setting temperature and then cooled in the tandem tube. The collected liquid was washed with aqueous NaOH and water, nearly colorless liquid was obtained.
References:
Yu, Zhi-Qun;Lv, Yan-Wen;Yu, Chuan-Ming;Su, Wei-Ke [Tetrahedron Letters,2013,vol. 54,# 10,p. 1261 - 1263]

591-35-5
236 suppliers
$10.00/1g

1435-46-7
131 suppliers
$8.00/1g
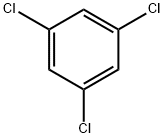
108-70-3
244 suppliers
$19.89/442235
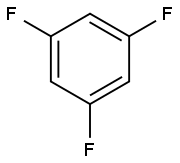
372-38-3
307 suppliers
$9.00/1g

1435-46-7
131 suppliers
$8.00/1g
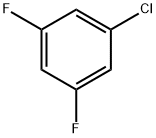
1435-43-4
222 suppliers
$7.00/5g