(3,5-Diisopropoxyphenyl)methanol synthesis
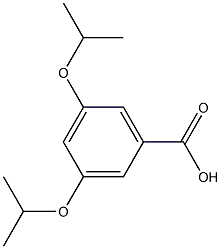
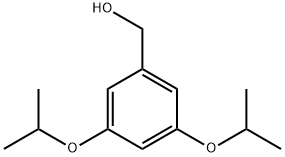
- Product Name:(3,5-Diisopropoxyphenyl)methanol
- CAS Number:94169-63-8
- Molecular formula:C13H20O3
- Molecular Weight:224.3
Yield:-
Reaction Conditions:
with diisobutylaluminium hydride in toluene at -78; for 0.75 h;Overall yield = 97 %;
Steps:
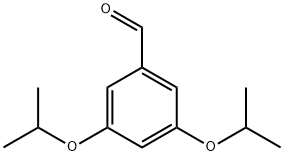
4.2.1. 3,5-Diisopropoxybenzaldehyde (2)
A 0.54 M solution of DIBAL-H in dichloromethane (4.66 mL, 2.52 mmol) was added to a solution of 3,5-diisopropoxybenzoic acid (282 mg, 1.01 mmol) in toluene (5 mL) at -78 °C. The reaction mixture was allowed to stir for 45 min at that temperature before quenching with 5% aqueous hydrochloric acid (1 mL). After warming to room temperature, the reaction mixture was diluted with water and the aqueous layer was extracted with ethyl acetate (3x5 mL), the combined organic layers were dried over magnesium sulfate, filtered and concentrated to yield a mixture of 3,5-diisopropoxybenzaldehyde and (3,5-diisopropoxyphenyl)methanol in 97% combined yield. 3,5-Diisopropoxybenzaldehyde: 1H NMR (400 MHz, CDCl3) δ (ppm): 9.77 (1H, s), 6.86 (2H, d, J = 2.31), 6.57 (1H, t, J = 2.31), 4.48 (2H, m, J = 6.15), 1.24 (6H,d, J = 6.16). 13C NMR (100 MHz, CDCl3) δ (ppm): 191.72, 159.44, 110.10, 108.33, 70.03, 21.72.
References:
St. John, Sarah E.;Jensen, Katherine C.;Kang, Soosung;Chen, Yafang;Calamini, Barbara;Mesecar, Andrew D.;Lipton, Mark A. [Bioorganic and Medicinal Chemistry,2013,vol. 21,# 19,p. 6022 - 6037]
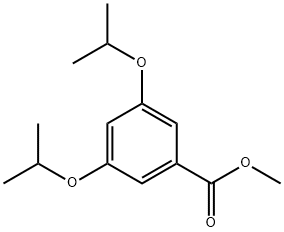
94169-62-7
36 suppliers
$56.54/100mgs:

94169-63-8
7 suppliers
inquiry
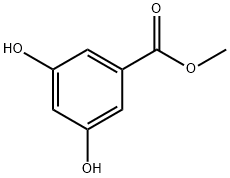
2150-44-9
262 suppliers
$5.00/5g

94169-63-8
7 suppliers
inquiry
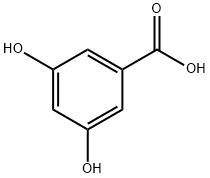
99-10-5
517 suppliers
$5.00/5g

94169-63-8
7 suppliers
inquiry