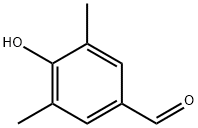
3,5-Dimethyl-4-hydroxybenzaldehyde synthesis
- Product Name:3,5-Dimethyl-4-hydroxybenzaldehyde
- CAS Number:2233-18-3
- Molecular formula:C9H10O2
- Molecular Weight:150.17
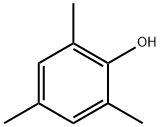
527-60-6
194 suppliers
$50.00/25g

2233-18-3
231 suppliers
$6.00/5g
Yield:2233-18-3 88%
Reaction Conditions:
with oxygen;cobalt(II) diacetate;sodium hydroxide in lithium hydroxide monohydrate;ethylene glycol at 50; under 760.051 Torr; for 12 h;Green chemistry;chemoselective reaction;Reagent/catalyst;Solvent;Time;
Steps:
2.2. Typical procedure for the Co(OAc)2-catalyzed aerobic oxidation of 1 (Table 2 in the text)
Typical procedure: a mixture of substrate 1 (5.0 mmol), Co(OAc)2*4H2O (0.05 mmol, 12 mg) and NaOH (5.0 mmol, 0.2 g) in EG/H2O (5.0 mL/0.25 mL) was stirred with O2 (1.0 atm) being bubbled at 50 °C for 12 h. Hydrochloric acid (10.0 mL, 2 %) and chloroform (10.0 mL) were successively added to the reaction mixture. The chloroform phase was separated, and the aqueous phase was further extracted with chloroform (10.0 mL × 2). The combined organic layer was dried over anhydrous sodium sulfate and concentrated to give a residue, which was purified by column chromatography on silica gel (eluents: .petroleum ether/ethyl acetate, 5/1) to provide the desired products 2. 3,5-Dimethyl-4-hydroxybenzaldehyde (2a):[1,2] white solid, 0.66 g (88% yield). Thespectral data see 2.1 section.
References:
Jiang, Jian-An;Du, Jia-Lei;Zhang, Zhong-Nan;Zhai, Jiao-Jiao;Ji, Ya-Fei [Synthetic Communications,2014,vol. 44,# 10,p. 1430 - 1440] Location in patent:supporting information

576-26-1
343 suppliers
$10.00/25g
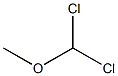
4885-02-3
191 suppliers
$10.00/1g

2233-18-3
231 suppliers
$6.00/5g
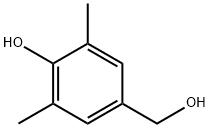
4397-14-2
50 suppliers
$50.00/250mg

2233-18-3
231 suppliers
$6.00/5g

5048-02-2
3 suppliers
inquiry

2233-18-3
231 suppliers
$6.00/5g