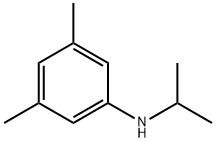
3,5-dimethyl-N-(propan-2-yl)aniline synthesis
- Product Name:3,5-dimethyl-N-(propan-2-yl)aniline
- CAS Number:52827-66-4
- Molecular formula:C11H17N
- Molecular Weight:163.26
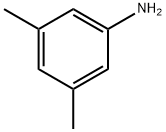
108-69-0
249 suppliers
$9.00/1g

52827-66-4
8 suppliers
$319.00/500mg
Yield:52827-66-4 8.2 g (34%)
Reaction Conditions:
with hydrogenchloride;sodium hydroxide;sodium tetrahydroborate;silica gel in methanol anol;acetone;
Steps:
1 Preparation of N-Isopropyl-3,5-dimethylaniline.
EXAMPLE 1 Preparation of N-Isopropyl-3,5-dimethylaniline. 3,5-Dimethylaniline (18.1 g, 0.15 mol) was dissolved in acetone (60 ml), the molecular sieves (30 g) added and then gently stirred at room temperature. After 24 hours, the sieves were removed by filtration and the excess acetone evaporated in vacuo. The resulting red oil was dissolved in methanol anol (75 ml), cooled to 10° C. and NaBH4 added in small increments (30 minutes). It was then allowed to warm to 20° C., acidified cautiously with 10% HCl and then made basic with approximately 20% NaOH solution and extracted with ether. The ether solution was dried over anhydrous magnesium sulfate and the ether removed in vacuo giving 20.0 g of a red oil. This oil was vacuum distilled yielding 8.2 g (34%) of the desired product boiling at 72°-74°/0.5 mm. Analysis calculated for NC11 H17: C, 80.92; H, 10.49; N, 8.58. Found: C, 80.67; H, 11.23; N, 7.72.
References:
US4196142,1980,A