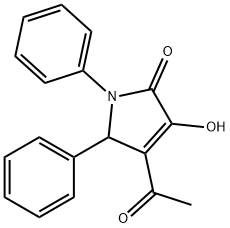
3-acetyl-4-hydroxy-1,2-diphenyl-2H-pyrrol-5-one synthesis
- Product Name:3-acetyl-4-hydroxy-1,2-diphenyl-2H-pyrrol-5-one
- CAS Number:113629-73-5
- Molecular formula:C18H15NO3
- Molecular Weight:293.32

100-52-7
977 suppliers
$20.37/250g

62-53-3
690 suppliers
$10.00/1g
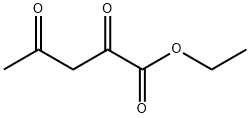
615-79-2
253 suppliers
$8.00/5g

113629-73-5
2 suppliers
inquiry
Yield:113629-73-5 80%
Reaction Conditions:
Stage #1: benzyl aldehyde;aminobenzenewith glacial acetic acid at 20; for 1 h;Inert atmosphere;
Stage #2: ethyl 2,4-diketopentanoate at 20; for 3 h;Inert atmosphere;Concentration;
Steps:
General procedure for the synthesis of 4-acetyl-3-hydroxy-3-pyrrolin-2-ones 4a-c
General procedure: To around-bottomed flask equipped with a mechanical stirrer was added benzaldehyde (1.5 equiv),aniline (1.0 equiv), and glacial acetic acid. The mixture was stirred at room temperature for 1 hour under Ar atmosphere and the imine formation was monitored by TLC (hexane/EtOAc5:1). Then, ethyl 2,4-dioxovalerate (1.0 equiv) was added, the resulting solution was stirredvigorously at room temperture under Ar atmosphere for 2-4 hours, and the formation of 3-hydroxy-3-pyrrolin-2-one derivatives was followed by TLC (CH2Cl2/CH3OH 5:0.2). Then, distilled water was added to the flask and the mixture was stirred for 15 minutes. The precipitate was filtered and then crude product was recrystallized from the solvent mixture of CH2Cl2 and toluene to obtain the pure compounds. 4-Acetyl-3-hydoxy-1,5-diphenyl-3-pyrrolin-2-one (4a): benzaldehyde (0.075 mL, 1.5 equiv,0. 75 mmol), aniline (0.046 mL, 1.0 equiv, 0.5 mmol), ethyl 2,4-dioxovalerate (0.07 mL,1.0 equiv, 0.5 mmol), and glacial acetic acid (1.0 mL), under Ar atmosphere. Reaction time is 3 h. The crude was purified via recrystallization from a solvent mixture of CH2Cl2 and tolueneto obtain 4a (117.6 mg, 80% yield) as a white solid. m.p. 222-223 °C.1H NMR (500 MHz, DMSO-d6) δ 7.58 (d, 3J(H,H) = 8 Hz, 2H; Ar-H), 7.29 (t, 3J(H,H) = 7.74Hz, 2H; Ar-H), 7.23 (d, 3J(H,H) = 7.42 Hz, 2H; Ar-H), 7.20 (t, 3J(H,H) = 7.32 Hz, 2H; Ar-H),7.15 - 7.08 (m, 2H; Ar-H), 2.34 ppm (s, 3H; CH3). 13C NMR (100 MHz, DMSO-d6) δ 164.58,136.93, 136.20, 128.67, 128.10, 127.64, 125.39, 122.49, 120.53, 60.46, 39.11 ppm. HRMS(ESI-quadrupole) m/z [M + H]+ calcd for C18H15NO3: 294.1130; found: 294.1120
References:
Nguyen, Nguyen Tran;Dai, Vo Viet;Tri, Nguyen Ngoc;Van Meervelt, Luc;Trung, Nguyen Tien;Dehaen, Wim [Beilstein Journal of Organic Chemistry,2022,vol. 18,p. 1140 - 1153] Location in patent:supporting information
![2,3-Pyrrolidinedione, 4-[1-(diphenylmethoxy)ethylidene]-1,5-diphenyl-](/CAS/20210305/GIF/113612-92-3.gif)
113612-92-3
0 suppliers
inquiry

113629-73-5
2 suppliers
inquiry
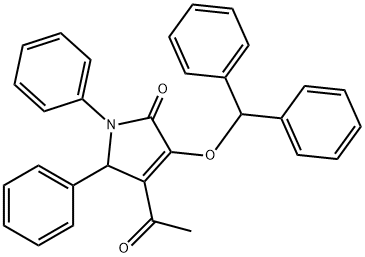
113612-90-1
0 suppliers
inquiry

113629-73-5
2 suppliers
inquiry