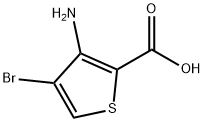
3-amino-4-bromothiophene-2-carboxylic acid synthesis
- Product Name:3-amino-4-bromothiophene-2-carboxylic acid
- CAS Number:215927-34-7
- Molecular formula:C5H4BrNO2S
- Molecular Weight:222.06
161833-42-7
99 suppliers
$11.00/100mg

215927-34-7
13 suppliers
inquiry
Yield:215927-34-7 97%
Reaction Conditions:
with lithium hydroxide monohydrate in methanol;water at 80; for 4 h;
Steps:
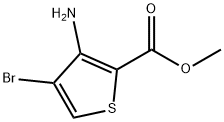
3-Amino-4-bromo-2-carboxythiophene (20):
To 3-amino-4-bromo-2-methoxycarbonylthiophene (5, 4.00g , 16.9 mmol) in methanol (17.5mL) at room temperature was added a solution of lithium hudroxide monohydrate(1.06 g, 25.3 mmol) in distilled water (70 mL), and the resulting mixture was stirred for 4 h at 80 oC. After completion of the reaction, the reaction mixture was adjusted to pH 3-4 by adding 1N hydrochloric acid and was partitioned between ethyl acetate and water. The aqueous layer extracted with ethyl acetate once more and the combined organic layer was dried over magnesium sulfate. The solvent was evaporated in vacuo to give the desired product 20 (3.60 g, 97%). 1H NMR (300 MHz, DMSO-d6): δ=7.80 (s, 1H), 6.29 ppm (br s, 2H).
References:
Jeon, Moon-Kook;Kim, Jung-Gyu;Lee, Duck-Hyung [Bulletin of the Korean Chemical Society,2016,vol. 37,# 9,p. 1406 - 1414] Location in patent:supporting information

22288-78-4
385 suppliers
$11.00/5g

215927-34-7
13 suppliers
inquiry