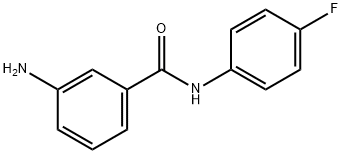
3-AMINO-N-(4-FLUOROPHENYL)BENZAMIDE synthesis
- Product Name:3-AMINO-N-(4-FLUOROPHENYL)BENZAMIDE
- CAS Number:251446-38-5
- Molecular formula:C13H11FN2O
- Molecular Weight:230.24

33489-69-9
15 suppliers
$15.00/5mg

251446-38-5
10 suppliers
$70.00/1g
Yield:251446-38-5 76%
Reaction Conditions:
with iron;ammonium chloride in methanol; for 7 h;Reflux;
Steps:
3.1.8. General Experimental Procedures of 4ii-8ii
General procedure: To a solution of 4i-8i (3.37 mmol) in methanol (20 mL) was added: ammonium chloride (doubleamounts of 4i-8i) and iron powder (6.03 mmol) and refluxed for 7 h. The reaction mixture wasevaporated to give an oily residue, which was added with water and extracted with dichloromethane(30 mL x 3). The combined organic phase was dried with anhydrous MgSO4, filtrated, and the filtratewas evaporated to yield a crude solid, which recrystallized with ethyl acetate and n-hexane to give apure white or pale yellow solid.
References:
Lee, Soo Hyun;Lee, Wonhwa;Bae, Jong-Sup;Ma, Eunsook [Molecules,2016,vol. 21,# 5,art. no. 676]
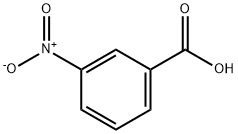
121-92-6
560 suppliers
$6.00/25g

251446-38-5
10 suppliers
$70.00/1g
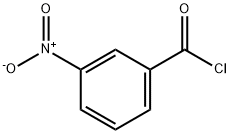
121-90-4
5 suppliers
$24.00/25g

251446-38-5
10 suppliers
$70.00/1g
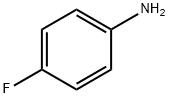
371-40-4
405 suppliers
$5.00/5G

251446-38-5
10 suppliers
$70.00/1g
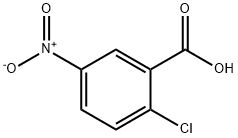
2516-96-3
442 suppliers
$6.00/25g

251446-38-5
10 suppliers
$70.00/1g