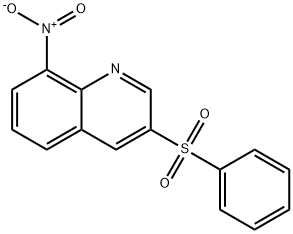
3-Benzenesulfonyl-8-nitro-quinoline synthesis
- Product Name:3-Benzenesulfonyl-8-nitro-quinoline
- CAS Number:607743-07-7
- Molecular formula:C15H10N2O4S
- Molecular Weight:314.32
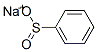
873-55-2
389 suppliers
$10.00/5g
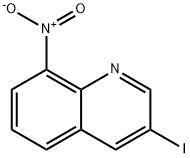
497084-46-5
43 suppliers
$60.00/100mg

607743-07-7
13 suppliers
inquiry
Yield:607743-07-7 58%
Reaction Conditions:
with copper(I) trifluoromethanesulfonate benzene in DMF (N,N-dimethyl-formamide) at 65; for 18 h;
Steps:
4
3-LODO-8-NITROQUINOLINE (D3) (135 g, 0.45 mol), was suspended in DIMETHYLFORMAMIDE (2.4 L) in a 5 L 3-necked flask fitted with an overhead stirrer, under an argon atmosphere. This mixture was treated successively with anhydrous sodium phenylsulfinate (99.6 g 0.608 mol), and BIS- (COPPER (I) TRIFLATE) benzene complex (170 g, 0.338 mol). The resulting SLURRY was heated to 65 °C for 18 h. The mixture was cooled, filtered and the solvent evaporated IN VACUO. Acetone (2.5 L) was added to the residue and the solution filtered. The filtrate was evaporated in vacuo, a further 2.5 L of acetone added and the mixture filtered again. The solvent was evaporated in vacuo and the residue dissolved in chloroform (3 L) and washed with 10% aqueous ammonia (2 x 2 L), and the organic phase was dried over magnesium sulfate and the solvent evaporated in vacuo. The dark brown residue was purified using a Biotage flash-150 chromatography apparatus (5 kg silica gel) eluting with hexane and increasing proportions of ethyl acetate to give the title compound (D4) (81.5 g, 58%) as a yellow solid ; BH (d6-DMSO) 7.67 (2H, t), 7.57 (1H, d, 7.96 (1H, t), 8.13 (2H, d), 8.51 (1H, d), 8.59 (1H, d), 9.42 (1H, d), 9.50 (1H, d); Mass Spectrum : C15H10SO4N2 requires 314; found 315 (MH+).
References:
WO2005/26125,2005,A1 Location in patent:Page/Page column 16-17
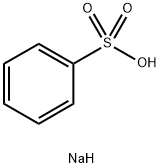
515-42-4
332 suppliers
$6.00/25g

497084-46-5
43 suppliers
$60.00/100mg

607743-07-7
13 suppliers
inquiry