
3'-(BENZYLOXY)[1,1'-BIPHENYL]-3-CARBONITRILE synthesis
- Product Name:3'-(BENZYLOXY)[1,1'-BIPHENYL]-3-CARBONITRILE
- CAS Number:893736-89-5
- Molecular formula:C20H15NO
- Molecular Weight:285.34

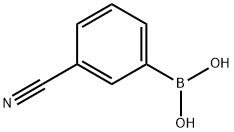
53087-13-1
205 suppliers
$11.00/5g
150255-96-2
354 suppliers
$8.00/1g
![3'-(BENZYLOXY)[1,1'-BIPHENYL]-3-CARBONITRILE](/StructureFile/ChemBookStructure8/GIF/CB7204348.gif)
893736-89-5
10 suppliers
inquiry
Yield:893736-89-5 60%
Reaction Conditions:
with barium dihydroxide;tetrakis(triphenylphosphine) palladium(0) in 1,2-dimethoxyethane;water at 80; for 6 h;
Steps:
6
A degassed mixture of 3-benzyloxy-phenyl bromide (28) (0.2 g, 0.76 mmol), 3- cyanophenylboronic acid (0.223 g, 1.52 mmol), barium hydroxide (0.285 g, 1.67 mmol), Pd(PPh3)4 (0.088 g, 0.076 mmol), DME (5 mL) and H2O (3 mL) was heated (800C) for 6 hours with vigorous stirring under an argon atmosphere. The reaction mixture was cooled to room temperature, diluted with ethyl acetate, and filtered through a plug of celite. The filtrate was diluted with brine; the organic phase was separated, dried (MgSO4) and concentrated in vacuo. The residue obtained was purified by flash column chromatography (20% diethyl? ether-hexane) to give 75 (0.130 g, 60% yield) as a viscous liquid. 1H NMR (500 MHz, CDCl3) δ 7.85 (t, J = 2.5 Hz, IH), 7.78 (dt, J = 7.5 Hz, J = 1.5 Hz, IH), 7.63 (dt, J = 8.0 Hz, J = 1.5 Hz, IH), 7.53 (t, J = 8.0 Hz, IH), 7.46 (d, J = 7.5 Hz, 2H)5 7.44- 7.37 (m, 3H)5 7.35 (t, J = 7.0 Hz, IH), 7.18-7.14 (m, 2H), 7.02 (dd, J = 8.5 Hz5 J = 2.5 Hz, IH)5 5.17 (s, 2H).
References:
WO2008/13963,2008,A2 Location in patent:Page/Page column 81