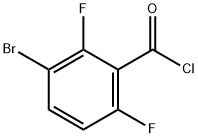
3-Brom-2.6-difluor-benzoesaeurechlorid synthesis
- Product Name:3-Brom-2.6-difluor-benzoesaeurechlorid
- CAS Number:1263376-89-1
- Molecular formula:C7H2BrClF2O
- Molecular Weight:255.44
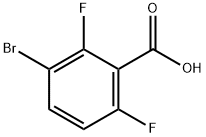
28314-81-0
114 suppliers
$7.00/100mg

1263376-89-1
7 suppliers
inquiry
Yield:-
Reaction Conditions:
with oxalyl dichloride;N,N-dimethyl-formamide in tetrahydrofuran at 10 - 30; for 2 h;
Steps:
67.2
(2) N-benzyl-3-bromo-2,6-difluoro-N-(2-hydroxyethyl)benzamide; To a solution of 3-bromo-2,6-difluorobenzoic acid (2.00 g, 8.44 mmol) and N,N-dimethylformamide (0.1 ml) in tetrahydrofuran (40 ml) was added dropwise oxalyl chloride (1.47 ml, 16.9 mmol) in a water bath, and the mixture was stirred at room temperature for 2 hr. The solvent was evaporated under reduced pressure. The residue was added under ice-cooling to a solution of N-benzylethanolamine (1.28 ml, 8.44 mmol) and triethylamine (1.77 ml, 12.7 mmol) in tetrahydrofuran (50 ml), and the mixture was stirred at 0°C for 15 min and at room temperature for 1 hr. The reaction mixture was poured into ice water, and the mixture was extracted with ethyl acetate. The extract was washed with water and dried over anhydrous magnesium sulfate. The solvent was evaporated under reduced pressure. The residue was recrystallized from a mixed solution of diethyl ether and diisopropyl ether to give the desired product (2.10 g, 67.3%) as a solid. 1H-NMR (CDCl3) δ; 3.28-3.84 (5H, m), 4.51 (1H, m), 4.81-4.99 (1H, m), 6.85-6.93 (1H, m), 7.15-7.37 (5H, m), 7.51-7.60 (1H, m).
References:
EP2123644,2009,A1 Location in patent:Page/Page column 78