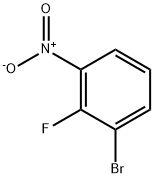
3-BROMO-2-FLUORONITROBENZENE synthesis
- Product Name:3-BROMO-2-FLUORONITROBENZENE
- CAS Number:58534-94-4
- Molecular formula:C6H3BrFNO2
- Molecular Weight:220
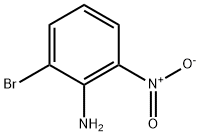
59255-95-7
131 suppliers
$28.00/1g

58534-94-4
188 suppliers
$10.00/1g
Yield:58534-94-4 52.7%
Reaction Conditions:
Stage #1: 2-bromo-6-nitroanilinewith hexafluorophosphoric acid in ethanol;water at 0 - 15; for 2 h;
Stage #2: with isopentyl nitrite in ethanol;water at 0 - 5; for 2 h;
Stage #3: in 5,5-dimethyl-1,3-cyclohexadiene at 120 - 130; for 2 h;Reagent/catalyst;Solvent;Temperature;
Steps:
7-9 Example 8 Preparation of 3-bromo-2-fluoronitrobenzene
Add 2-bromo-6-nitroaniline (21.7g, 0.1mol) to a 500mL reaction flask,Anhydrous ethanol (200 mL) was added.The temperature was lowered from 0 to 10 ° C, and an aqueous solution of hexafluorophosphoric acid (40%) (44.9 g) was added dropwise.Control temperature is 10-15 . After the addition was completed, the temperature was maintained for 2 hours.The temperature was lowered to 0 ° C, and isoamyl nitrite (14.1 g, 0.12 mol) was added dropwise. After the dropwise addition was completed,Reaction at 0-5 ° C for 2h. Filtration and washing with absolute ethanol (20 mL) gave hexafluorophosphate.Xylene (200 mL) was added to a 500 mL reaction flask, and the temperature was raised to 120 to 130 ° C.Add the hexafluorophosphate obtained in the previous step and incubate for 2h. Cool to room temperature,The reaction solution was poured into a saturated sodium carbonate solution, the layers were separated, and the organic phase was dried over anhydrous sodium sulfate.Distillation under reduced pressure gave 11.6 g of a pale yellow liquid. The yield was 52.7%,HPLC purity was 99.1%.
References:
CN110305018,2019,A Location in patent:Paragraph 0071-0088
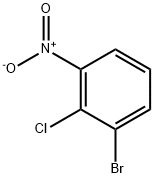
3970-37-4
127 suppliers
$11.00/250mg

58534-94-4
188 suppliers
$10.00/1g
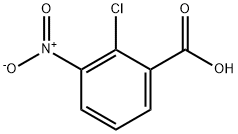
3970-35-2
251 suppliers
$6.00/250mg

58534-94-4
188 suppliers
$10.00/1g
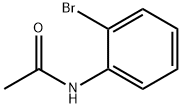
614-76-6
130 suppliers
$9.00/1g

58534-94-4
188 suppliers
$10.00/1g
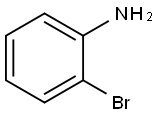
615-36-1
406 suppliers
$10.00/5g

58534-94-4
188 suppliers
$10.00/1g