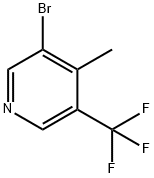
3-Bromo-4-methyl-5-(trifluoromethyl)pyridine synthesis
- Product Name:3-Bromo-4-methyl-5-(trifluoromethyl)pyridine
- CAS Number:1211518-50-1
- Molecular formula:C7H5BrF3N
- Molecular Weight:240.02
436799-33-6
197 suppliers
$6.00/250mg

74-88-4
357 suppliers
$15.00/10g

1211518-50-1
5 suppliers
inquiry
Yield:1211518-50-1 17.92%
Reaction Conditions:
Stage #1: 3-bromo-5-(trifluoromethyl)pyridinewith n-butyllithium;diisopropylamine in tetrahydrofuran at -100 - -10;
Stage #2: methyl iodide in tetrahydrofuran at -78; for 0.5 h;
Steps:
153
Example 153Preparation of 1-(benzo[d]thiazol-6-yl)-3-(4-methyl-5-(trifluoromethyl)pyridin-3-yl)imidazolidin-2-one, Trifluoroacetic Acid Salt (153A) Preparation of Starting Material 3-Bromo-4-methyl-5-trifluoromethyl-pyridine (SM-153a)Butyl Lithium (1.9 mL, 3.044 mmol) was added to a solution of DIPA (335.7 mg, 3.318 mmol) in THF (6 mL) at -78° C. The reaction mixture was stirred at -10° C. for 10 minutes. This was followed by the addition of 3-bromo-5-trifluoromethyl-pyridine (500 mg, 2.212 mmol) in THF (3 mL) at -100° C. The reaction mixture was stirred for a further 15 minutes at -90° C. and was followed by the addition of methyl iodide (557.0 mg, 3.924 mmol) in THF (2 mL) at -78° C. with stirring over a period of 30 minutes. The reaction was monitored by TLC (5% ethylacetate in hexane). The reaction mixture was quenched with aqueous NaHCO3 solution and extracted with ethylacetate (100 mL). The organic layer was dried over Na2SO4 and concentrated. Purification by column chromatography on silica gel (2% ethylacetate in hexane) afforded 95 mg of the product (17.92% yield).LCMS: m/z=239.8 (M+1)
References:
US2010/331326,2010,A1 Location in patent:Page/Page column 60