
3-BroMo-4-(Methylthio)toluene synthesis
- Product Name:3-BroMo-4-(Methylthio)toluene
- CAS Number:89981-02-2
- Molecular formula:C8H9BrS
- Molecular Weight:217.13
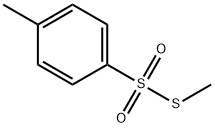
4973-66-4
24 suppliers
$28.60/25MG

854636-01-4
21 suppliers
inquiry

89981-02-2
11 suppliers
$55.00/250mg
Yield:89981-02-2 82%
Reaction Conditions:
with sodium hydrogencarbonate;copper(II) sulfate in methanol at 20; for 48 h;Inert atmosphere;
Steps:
A general procedure for the preparation of o-bromoaryl methyl sulfidesS1
General procedure: To a mixture of NaHCO3 (1.01 g, 12.0 mmol, 2.0 equiv) and CuSO4 (95.8 mg, 0.600 mmol, 10 mol %) wasadded a solution of S-methyl 4-toluenethiosulfonate (2) (1.21 g, 6.00 mmol) and 2-bromophenylboronic acid (1a)(1.81 g, 9.00 mmol, 1.5 equiv) dissolved in MeOH (40 mL) at room temperature. After stirring for 48 h at thesame temperature, to the mixture was added an aqueous saturated ammonium chloride solution (10 mL). Themixture was extracted with EtOAc (50 mL 3), and the combined organic extract was washed with brine (20 mL),dried (Na2SO4), and after filtration, the filtrate was concentrated under reduced pressure. The residue was purifiedby flash column chromatography (silica-gel 30 g, n-hexane) to give 2-bromophenyl methyl sulfide (867 mg, 4.27mmol, 71.1%) as a colorless oil.
References:
Matsuzawa, Tsubasa;Uchida, Keisuke;Yoshida, Suguru;Hosoya, Takamitsu [Chemistry Letters,2018,vol. 47,# 7,p. 825 - 828] Location in patent:supporting information

623-13-2
111 suppliers
$33.39/5g

89981-02-2
11 suppliers
$55.00/250mg
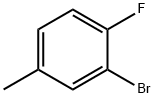
452-62-0
272 suppliers
$6.00/5g
5188-07-8
287 suppliers
$13.00/5g

89981-02-2
11 suppliers
$55.00/250mg