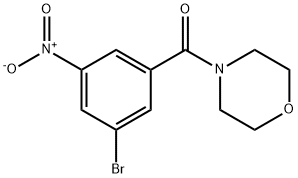
(3-Bromo-5-nitrophenyl)(morpholino)methanone synthesis
- Product Name:(3-Bromo-5-nitrophenyl)(morpholino)methanone
- CAS Number:941294-19-5
- Molecular formula:C11H11BrN2O4
- Molecular Weight:315.12

110-91-8
677 suppliers
$9.00/1g
6307-83-1
210 suppliers
$5.00/250mg

941294-19-5
30 suppliers
$75.00/1g
Yield:-
Reaction Conditions:
with N-ethyl-N,N-diisopropylamine;HATU in N,N-dimethyl-formamide at 0; for 2 h;
Steps:
1.29.1
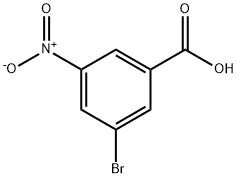
Step 1 : HATU (927 mg, 2.44 mmol), morpholine (0.212 mL, 2.44 mmol), and diisopropylethylamine (0.532 mL, 3.05 mmol) were added sequentially to a solution of 3-bromo- 5-nitrobenzoic acid (500 mg, 2.03 mmol) in DMF (10 mL) at 0 °C. The rection mixture was stirred for 2 hours at 0 °C, then the mixture was diluted with ethyl acetate (50 mL) and washed with saturated aqueous aHC03 (50 mL) followed by 1 : 1 water: brine (2 x 30 mL). The organic layers were dried over sodium sulfate, filtered, and concentrated in vacuo. The residue was purified by silica gel column chromatography (ethyl acetate/hexanes) provided (3-bromo-5- nitrophenyl)(morpholin-4-yl)methanone as a white solid. XH NMR (400 MHz, CDC13) δ 8.43 (t, J= 1.9 Hz, 1H), 8.28 - 8.12 (m, 1H), 7.89 (t, J= 1.5 Hz, 1H), 3.74 (br m, 8H).
References:
WO2014/31438,2014,A2 Location in patent:Paragraph 00225