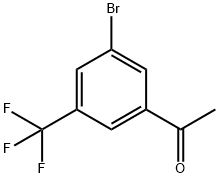
3'-BroMo-5'-(trifluoroMethyl)acetophenone, 97% synthesis
- Product Name:3'-BroMo-5'-(trifluoroMethyl)acetophenone, 97%
- CAS Number:154259-25-3
- Molecular formula:C9H6BrF3O
- Molecular Weight:267.04
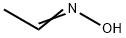
107-29-9
201 suppliers
$9.00/1g
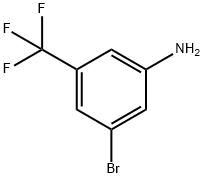
54962-75-3
299 suppliers
$5.00/1g

154259-25-3
51 suppliers
$21.00/100mg
Yield:154259-25-3 51%
Reaction Conditions:
Stage #1:[3-bromo-5-(trifluoromethyl)phenyl]amine with hydrogenchloride;sulfuric acid;sodium nitrite in water at 0; for 0.5 h;
Stage #2:Acetaldehyde oxime with copper(II) sulfate in water at 20; for 4 h;Heating / reflux;
Steps:
1-(3-Bromo-5-(trifluoromethyl)phenyl)ethanone. A flask was charged with water (42 ml), cooled to 0° C., and treated with concentrated hydrochloric acid (21.7 ml) and sulfuric acid (5.66 ml). To this was added 3-amino-5-bromobenzotrifluoride (8.77 ml, 62.5 mmol). The reaction was treated with a solution of sodium nitrite (5.39 g, 78 mmol) in water (10 mL). The resulting reaction mixture was stirred for 30 min at 0° C. The reaction was transferred to a solution of acetaldoxime (5.71 ml, 94 mmol) and copper(II) sulfate (0.499 g, 3.12 mmol) in water (30 mL) at room temperature. After stirring for 1 h at room temperature, the reaction was heated to reflux and held there for 3 h. The reaction was cooled and diluted with pentane. It gave an intractable suspension. The reaction mixture was filtered through a sintered glass funnel. The layers were separated. The organics were washed with water, then brine, dried over magnesium sulfate, and concentrated. The crude residue was distilled (high vacuum, 75° C.) to give 3 fractions of varying levels of purity. Total yield was 8.5 g (51%) with purity that ranged from 10:1 to 1:1. 1H NMR (500 MHz, CDCl3) δ ppm 8.25 (s, 1H), 8.11 (s, 1H), 7.95 (s, 1H), 2.63 (s, 3H).
References:
Bristol-Myers Squibb Company US2007/249607, 2007, A1 Location in patent:Page/Page column 76
880652-44-8
15 suppliers
inquiry

75-16-1
266 suppliers
$12.00/10ml

154259-25-3
51 suppliers
$21.00/100mg

401-84-3
128 suppliers
$6.00/250mg
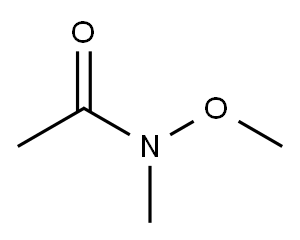
78191-00-1
268 suppliers
$6.00/5g

154259-25-3
51 suppliers
$21.00/100mg
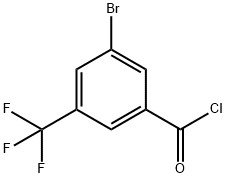
958258-15-6
11 suppliers
inquiry
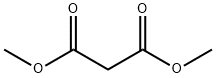
108-59-8
578 suppliers
$5.00/25g

154259-25-3
51 suppliers
$21.00/100mg
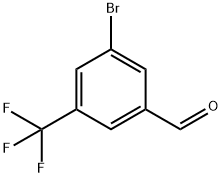
477535-41-4
135 suppliers
$15.00/1g

154259-25-3
51 suppliers
$21.00/100mg