
3-broMo-6-hydroxy-2-Methylbenzaldehyde synthesis
- Product Name:3-broMo-6-hydroxy-2-Methylbenzaldehyde
- CAS Number:137644-94-1
- Molecular formula:C8H7BrO2
- Molecular Weight:215.04
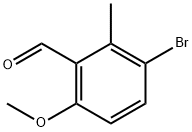
137644-93-0
7 suppliers
inquiry

137644-94-1
17 suppliers
inquiry
Yield:137644-94-1 97.6%
Reaction Conditions:
with BBr3 in dichloromethane at 0; for 1.5 h;Inert atmosphere;
Steps:
17.2 17.2 Preparation of 3-bromo-6-hydroxy-2-methylbenzaldehyde
A solution of boron tribromide (13.1 g, 52.4 mmol, 5.1 mL, 1 eq) in dichloromethane (5 ml) was added to a stirred solution of 3-bromo-6-methoxy-2-methyl-benzaldehyde (12 g, 52.4 mmol, 1 eq) in DCM (150 mL) under nitrogen at 0°C. The reaction mixture was stirred for 1.5 hours at 0°C.TLC showed the reaction was completed. Water (400 mL) was added cautiously at 0°C and the mixture was continue stirred for 15 min. TLC showed the reaction was completed. The organic layer was washed with sodium bicarbonate aqueous (200 mL), water (200 mL) and brine (200 mL), then the organic layer was dried over sodium sulfate and concentrated under reduced pressure to give the crude product as brown solid which was purified by silica gel column chromatography (Petroleum ether/Ethyl acetate=100:1 to 50:1) to afford 3-bromo-6-hydroxy-2- methyl-benzaldehyde (11 g, 97.6% yield) as yellow solid.1H NMR (400 MHz, CDCl3) δ 12.07 (s, 1H), 10.37 (s, 1H), 7.64 (d, J = 8.8 Hz, 1H), 6.75 (d, J = 8.8 Hz, 1H), 2.69 (s, 3H).
References:
WO2022/133420,2022,A1 Location in patent:Paragraph 0251; 0253
54884-55-8
89 suppliers
$45.00/100mg

137644-94-1
17 suppliers
inquiry

6161-65-5
157 suppliers
$11.00/250mg

137644-94-1
17 suppliers
inquiry
79383-44-1
61 suppliers
$80.00/1g

137644-94-1
17 suppliers
inquiry
89244-39-3
14 suppliers
inquiry

137644-94-1
17 suppliers
inquiry