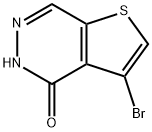
3-bromo-Thieno[2,3-d]pyridazin-4(5H)-one synthesis
- Product Name:3-bromo-Thieno[2,3-d]pyridazin-4(5H)-one
- CAS Number:1433203-93-0
- Molecular formula:C6H3BrN2OS
- Molecular Weight:231.07
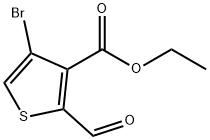
1433203-92-9
6 suppliers
inquiry
![3-bromo-Thieno[2,3-d]pyridazin-4(5H)-one](/CAS/20180703/GIF/1433203-93-0.gif)
1433203-93-0
12 suppliers
inquiry
Yield:1433203-93-0 59%
Reaction Conditions:
with hydrazine in tetrahydrofuran;ethanol at 80; for 2 h;
Steps:
335.3 Ste 3: (0642) Preparation of 3-bromothieno[2,3-d]pyridazin-4(5H)-one
Ste 3: (0642) Preparation of 3-bromothieno[2,3-d]pyridazin-4(5H)-one: (0643) [00260] To a solution of ethyl 4-bromo-2-formylthiophene-3-carboxylate (1.56 g, 5.93 mmol) in ethanol (10 mL) was added hydrazine solution (1M in tetrahydofuran solution). The reaction mixture was heated to 80 °C and stirred for 2h. The mixture was allowed to cool to room temperature, the precipitated product was filtered, washed with dichloromethane and dried to obtain title compound 3-bromothieno[2,3-d]pyridazin-4(5H)-one (0.81 g, 59% yield) as a white solid. Calculated (M+H): 230.91; Found (M+H): 230.9
References:
WO2017/100591,2017,A1 Location in patent:Paragraph 00260
16694-17-0
165 suppliers
$11.00/250mg
![3-bromo-Thieno[2,3-d]pyridazin-4(5H)-one](/CAS/20180703/GIF/1433203-93-0.gif)
1433203-93-0
12 suppliers
inquiry
1334640-34-4
8 suppliers
inquiry
![3-bromo-Thieno[2,3-d]pyridazin-4(5H)-one](/CAS/20180703/GIF/1433203-93-0.gif)
1433203-93-0
12 suppliers
inquiry