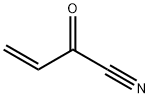
3-Butenoic acid, 2-oxo-, methyl ester synthesis
- Product Name:3-Butenoic acid, 2-oxo-, methyl ester
- CAS Number:108736-44-3
- Molecular formula:C5H6O3
- Molecular Weight:114.1
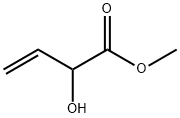
5837-73-0
45 suppliers
$45.00/25mg

108736-44-3
0 suppliers
inquiry
Yield:108736-44-3 94.4%
Reaction Conditions:
with tris(triphenylphosphine)ruthenium(II) chloride;tert-amyl hydroperoxide in toluene at 0 - 23; for 2 h;Inert atmosphere;
Steps:
2 example 2: Synthesis of methyl 2-oxo-3-butenoate
Add 3 g (24.0 mmol) of acrylonitrile acetate to a 250 mL three-necked flask, add 100 mL of saturated hydrogen chloride methanol solution, stir to dissolve, heat and reflux at 75 ° C for 2 hours, transfer to a single-mouth bottle, and remove hydrogen chloride by rotary distillation. Methanol gives the compound methyl 2-hydroxy-3-butenoate;Methyl 2-hydroxy-3-butenoate was dissolved in 50 mL of toluene and transferred to a three-neck bottle.0.11 g (0.12 mmol) of tris(triphenylphosphine) ruthenium dichloride was added under nitrogen.The system was cooled to 0 ° C, and the tetraamyl hydrogen peroxide was added dropwise with a constant pressure dropping funnel.6.24g (48mmol, 80% content) of 50mL in toluene solution,The drip acceleration maintains the internal temperature of the reaction not exceeding 23 ° C.After about 1 hour, the addition was completed, and after the completion of the dropwise addition, the internal temperature was maintained at 23 ° C for 1 hour.Excessive p-pentyl hydroperoxide was quenched by adding 3 g (24 mmol) of sodium sulfite, stirred for 10 min, filtered, and the filtrate was steamed at not more than 30 ° C and a vacuum of 3.9 KPa to obtain methyl 2-oxo-3-butenoate. The ester was 2.9 g, the GC purity was 96.5%, and the yield was 94.4%.
References:
CN109912416,2019,A Location in patent:Paragraph 0052; 0053
553-90-2
324 suppliers
$10.00/5g
3536-96-7
207 suppliers
$67.00/100ml

108736-44-3
0 suppliers
inquiry