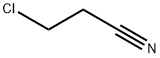
3-Chloropropiononitrile synthesis
- Product Name:3-Chloropropiononitrile
- CAS Number:542-76-7
- Molecular formula:C3H4ClN
- Molecular Weight:89.52

98-88-4
592 suppliers
$10.00/5g

109-78-4
377 suppliers
$10.00/1g

542-76-7
145 suppliers
$31.00/25mL

5325-95-1
6 suppliers
inquiry
Yield:542-76-7 75%
Reaction Conditions:
with 1-pyrrolidinecarboxaldehyde at 80; for 5.5 h;Reagent/catalyst;Temperature;
Steps:
General procedure: Entry 1: According to general procedure Ill (chapter 2.1.3) a 100 mL flask with strong stir bar was charged with 3-hydroxypropannitrile 145 (14.1 mL, 14.66 g, 200 mmol, 1.0 equiv) and FPyr (7.9 mL, 8.10 g, 80.0 mmol, 40mo1%) and heated to 80 °C. Within 1.25 h benzoyl chloride (23.9 mL, 28.97 g,204 mmol, 1.02 equiv) was added dropwise and the reaction mixture was stirred for further 4.25 h at80 °C. After 4 h of stirring ‘H-N MR of a small aliquot of the reaction mixture (ca. 10 mg) showed fullconsumption of the starting material 145 and a chloride 245 to ester 345 ratio of 82:18. Then thereaction solution was allowed to cool down to ambient temperature accompanied by benzoic acidprecipitating. Distillation at 10 mbar through a micro distillation apparatus without dispenser delivered the chloride 245 as a colorless oil (13.75 g) with a boiling range of 66-80 °C (oil bath temperature 100-150 °C). As ‘H-N MR showed 2 mol% formiate 745 (referred to 245), the desired chloroalkane 245 was obtained in 75% yield (150.2 mmol).
References:
UNIVERSITAET DES SAARLANDES;HUY, Peter Helmut WO2016/202894, 2016, A1 Location in patent:Page/Page column 53; 132; 142; 143

107-13-1
420 suppliers
$16.00/25ML

542-76-7
145 suppliers
$31.00/25mL
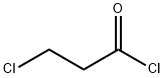
625-36-5
379 suppliers
$45.00/100ml

542-76-7
145 suppliers
$31.00/25mL

109-78-4
377 suppliers
$10.00/1g

542-76-7
145 suppliers
$31.00/25mL
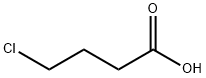
627-00-9
224 suppliers
$49.00/10g

542-76-7
145 suppliers
$31.00/25mL