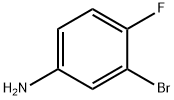
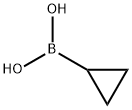
3-cyclopropyl-4-fluoroaniline synthesis
- Product Name:3-cyclopropyl-4-fluoroaniline
- CAS Number:890129-90-5
- Molecular formula:C9H10FN
- Molecular Weight:151.1808
Yield:890129-90-5 99.35%
Reaction Conditions:
with potassium phosphate;palladium diacetate;triphenylphosphine in toluene at 100; for 16 h;Inert atmosphere;
Steps:
18 Step 1: Synthesis of Compound BB-18
The compounds BB-1-4 (1.00 g, 5.26 mmol, 1.00 eq), BB-18-1 (587.38 mg, 6.84 mmol, 1.30 eq), potassium phosphate (3.91 g, 18.41 mmol, 3.50 eq), triphenylphosphine (137.96 mg, 526.00 umol, 0.10 eq), and palladium acetate (59.05 mg, 263.00 umol, 0.05 eq) were dissolved in toluene (24.00 mL) and water (2.00 mL), heated to 100° C. in nitrogen ambient and reacted for 16 hours. After being heated, the reaction solution gradually turned dark brown from brown. Complete reaction of raw materials was observed by LCMS monitoring and a target compound was generated. The reaction solution was cooled to 23° C., followed by addition of 20 mL of water, extraction with ethyl acetate (20 mL*3), drying over anhydrous sodium sulfate, and filtration. A filtrate was dried by rotary evaporation under reduced-pressure distillation, and purified by flash silica gel column chromatography (petroleum ether:ethyl acetate=10:1). The reaction succeeded, and a yellow liquid product BB-18 (790.00 mg, yield: 99.35%) was obtained. MS (ESI) m/z: 152 [M+H]+.
References:
US2019/169140,2019,A1 Location in patent:Paragraph 0155-0158