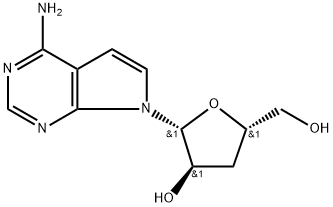
3'-Deoxytubercidin synthesis
- Product Name:3'-Deoxytubercidin
- CAS Number:40725-89-1
- Molecular formula:C11H14N4O3
- Molecular Weight:250.26
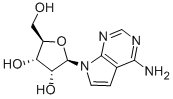
69-33-0
152 suppliers
$26.00/1mg

40725-89-1
15 suppliers
inquiry
Yield:40725-89-1 43%
Reaction Conditions:
Stage #1: tubercidinwith 2-acetoxy-2-methylpropanoyl chloride;sodium iodide in acetonitrile; for 1.5 h;
Stage #2: with palladium on activated charcoal;hydrogen in methanol;
Stage #3: with ammonia in methanol;
Steps:
General procedure 1 (conversion of ribo-nucleoside into 3'-deoxy analogue)
General procedure: Nal (10 eq.) was dissolved in anhydrous MeCN (10mL/mmol SM), and stirred for 5min under argon. Next, a-acetoxyisobutyrylchloride (3.5 eq.) was added, giving a white precipitate. The mixture was stirred vigorously for another 5 - 10min, after which the appropriate ribonucleoside (1 eq.) was added in one portion. The resulting mixture was stirred for 1 .5H after which TLC showed full conversion of SM. The mixture was poured in aq. sat. NaHC03 / aq. sat. Na2S203 solution. Next, CHCI3 was added, and the layers separated. The water layer was extracted with CHCI3 twice more. Organic layers were combined, dried over Na2S04, filtered and evaporated. The resulting oil was dissolved in EtOH (7.5 mL / mmol SM) and 1 M aq. NaOAc solution (2.5 mL / mmol SM) was added. Next, the flask was purged with N2, after which a cat. amount of Pd/C was added. Next, the N2- atmosphere was exchanged for H2 (balloon; no bubbling) and the mixture stirred overnight. Next, the mixture was purged with N2 to remover residual H2-gas, and filtered over a pad of Celite. The mixture was evaporated till dryness, and partitioned between EA and aq. sat. NaHC03 / aq. sat. Na2S203 solution. Layers were separated and the water layer extracted twice more with EA. Organic layers were combined, dried over Na2S04, filtered and evaporated. The resulting oil was dissolved in 7N NH3 in MeOH and stirred overnight. The solvent was removed, and the residue purified by column chromatography 0→ 15 % MeOH / DCM.
References:
WO2019/76633,2019,A1 Location in patent:Page/Page column 44; 47
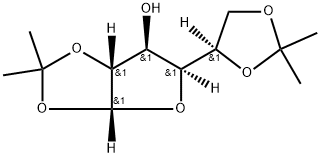
582-52-5
468 suppliers
$5.00/5g

40725-89-1
15 suppliers
inquiry
![3-(2,2-dimethyl-1,3-dioxolan-4-yl)-7,7-dimethyl-2,6,8-trioxabicyclo[3.3.0]octane](/CAS/GIF/4613-62-1.gif)
4613-62-1
28 suppliers
inquiry

40725-89-1
15 suppliers
inquiry
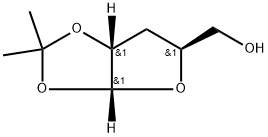
3396-71-2
5 suppliers
inquiry

40725-89-1
15 suppliers
inquiry
![[(3aR,5S,6aR)-2,2-dimethyltetrhydrofuro[2,3-d][1,3]dioxol-5-yl]methyl benzoate](/CAS/20180629/GIF/4105-29-7.gif)
4105-29-7
6 suppliers
inquiry

40725-89-1
15 suppliers
inquiry