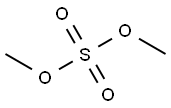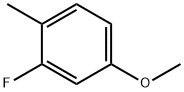
3-FLUORO-4-METHYLANISOLE synthesis
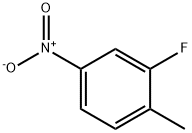
- Product Name:3-FLUORO-4-METHYLANISOLE
- CAS Number:405-06-1
- Molecular formula:C8H9FO
- Molecular Weight:140.15
Yield: 65%
Reaction Conditions:
with potassium carbonate in N,N-dimethyl-formamide at 20; for 16 h;Inert atmosphere;
Steps:
10 2-Fluoro-4-methoxy-1-methylbenzene
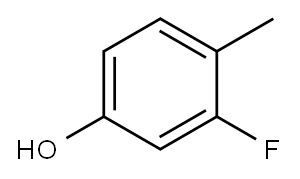
To a solution of 1.00 g (7.93 mmol) of 3-fluoro-4-methylphenol in 20 ml of dehydrated DMF, 3.30 g (23.9 mmol) of potassium carbonate and 0.750 ml (12.0 mmol) of methyl iodide were added at room temperature in an argon atmosphere and reacted at room temperature for 16 hours with stirring.
After completion of the reaction, water was added to the reaction solution, followed by extraction with ethyl acetate.
The obtained organic layer was washed with a saturated aqueous solution of sodium chloride, then dried over anhydrous magnesium sulfate, filtered, and concentrated under reduced pressure.
The obtained concentration residue was subjected to preparative column chromatography (apparatus 1, silica gel, elution solvent: n-hexane:ethyl acetate=100:0→80:20 (V/V)), and a fraction containing the compound of interest was concentrated under reduced pressure to obtain 725 mg of the title compound (yield: 65%) as a colorless oil.
Mass spectrum (CI, m/z): 141 [M+1]+.
1H-NMR spectrum (400 MHz, CDCl3) δ: 7.10-7.01 (m, 1H), 6.62-6.55 (m, 2H), 3.77 (s, 3H), 2.21-2.18 (m, 3H).
References:
UBE INDUSTRIES, LTD.;IWASE, Noriaki;AGA, Yasuhiro;USHIYAMA, Shigeru;KONO, Shigeyuki;SUNAMOTO, Hidetoshi;MATSUSHITA, Takashi;OGI, Sayaka;UMEZAKI, Satoshi;KOJIMA, Masahiro;ONUMA, Kazuhiro;SHIRAISHI, Yusuke;OKUDO, Makoto;KIMURA, Tomio US2018/186818, 2018, A1 Location in patent:Paragraph 1130; 1131; 1132; 1133; 1134
50868-72-9
152 suppliers
$7.00/250mg

405-06-1
124 suppliers
$16.00/1g
452-78-8
162 suppliers
$17.00/1g

405-06-1
124 suppliers
$16.00/1g
1427-07-2
353 suppliers
$6.00/5g

405-06-1
124 suppliers
$16.00/1g