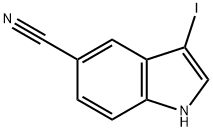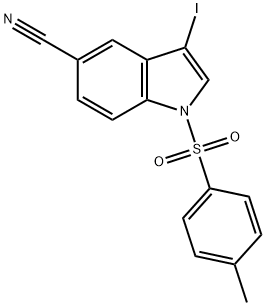
3-Iodo-1-tosyl-1h-indole-5-carbonitrile synthesis
- Product Name:3-Iodo-1-tosyl-1h-indole-5-carbonitrile
- CAS Number:676273-39-5
- Molecular formula:C16H11IN2O2S
- Molecular Weight:422.24
Yield:676273-39-5 95.5%
Reaction Conditions:
Stage #1: 3-iodo-1H-indole-5-carbonitrilewith sodium hydride in N,N-dimethyl-formamide;mineral oil at 0 - 20;
Stage #2: p-toluenesulfonyl chloride in mineral iol;N,N-dimethyl-formamide at 0 - 20;
Steps:
69
Preparation of compound 69b: 3-iodo-l-tosyl-l/7-indole-5-carbonitrileTo a solution of 3-iodo-l//-indole-5-carbonitrile (2 g, 7.40 mmol) in DMF (20 mL) was added 60% NaH (538 mg, 22.38 mmol) portion wise at 0 °C and the reaction was stirred for 10 min RT. To the above mixture at 0 °C, p-TsCl (2.2 g, 11.19 mmol) solution in DMF (4 mL) was added and stirred for further 2 h at RT. The reaction was quenched with ice cold H20 (20 mL). The resulting suspension was filtered and the solid was washed with H20 (10 mL) and dried. The crude was purified with silica gel chromatography (eluent: 20% EtOAc in petroleum ether) to afford 3-iodo-l-tosyl-l//-indole-5-carbonitrile (3.0 g, 95.5%) as an off brown solid. .H NMR (400MHz, DMSO-d6): δ 8.30 (s, 1H), 8.13 (d, J=8.8Hz, 1H), 7.98 (d, J=8.4Hz, 2H), 7.87-7.81 (m, 2H), 7.43 (d, J=8.0Hz, 2H), 2.32 (s, 3H).
References:
WO2012/129338,2012,A1 Location in patent:Page/Page column 193
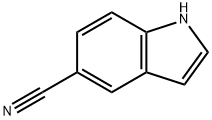
15861-24-2
434 suppliers
$10.00/5g

676273-39-5
36 suppliers
inquiry