
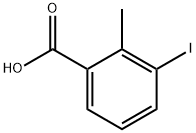
3-Iodo-2-methyl-benzaldehyde synthesis
- Product Name:3-Iodo-2-methyl-benzaldehyde
- CAS Number:848444-83-7
- Molecular formula:C8H7IO
- Molecular Weight:246.05

76350-89-5
37 suppliers
$45.00/50mg

848444-83-7
15 suppliers
inquiry
Yield:848444-83-7 87%
Reaction Conditions:
with 2,2,6,6-tetramethyl-piperidine-N-oxyl;sodium hypochlorite;sodium hydrogencarbonate;potassium bromide in dichloromethane;water at 0; for 0.45 h;
Steps:
P2
Alcohol 2 was treated with DCM (12 mL), TEMPO (19 mg, 0.12 MMOL), and KBR (0.71 g, 0.61 MMOL) and cooled to 0 °C. Then to this alcohol mixture was added a pre-mix solution of NAOCI (5 mL, 9.68 MMOL), NaHCO3 (0.60 g, 0.70 MMOL), H20 (5 mL) over 2 min. The heterogeneous mixture turned red and after stirring for 25 min, the layers were separated. The aqueous layer was extracted with DCM (3 x 50 mL), and the combined organic layers were washed with Brine (1 x 100 mL) and dried (MgS04). The crude product was purified by SiO2 column chromatography (1: 10-EtOAc: Hex) TO- give the aldehyde 3 (1.30 g, 87%). 1HNMR (300 MHZ, CDCL3) No. 10. 2 (s, 1H), 8.06 (d, J=7. 9HZ, 1H), 7.79 (d, J = 6.6 Hz, 1 H), 7.08 (t, J = 7.9 Hz, 1 H), 2.78 (s, 3H) ppm.
References:
WO2005/26114,2005,A1 Location in patent:Page/Page column 100-101
133232-56-1
139 suppliers
$12.00/1g

848444-83-7
15 suppliers
inquiry