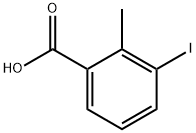
3-Iodo-2-methylbenzoic acid synthesis
- Product Name:3-Iodo-2-methylbenzoic acid
- CAS Number:133232-56-1
- Molecular formula:C8H7IO2
- Molecular Weight:262.05
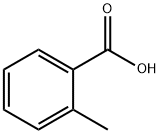
118-90-1
533 suppliers
$13.00/1g

133232-56-1
142 suppliers
$12.00/1g
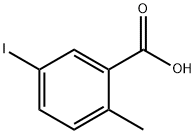
54811-38-0
406 suppliers
$5.00/5g
Yield: 92% , 0.7%
Reaction Conditions:
with hydrogen iodide;iodine;acetic anhydride;acetic acid;H-β-form zeolite at 122; for 4 h;Product distribution / selectivity;
Steps:
1; 15 [Example 1]
5-iodo-2-methylbenzoic acid: 99.7% (purity in crystals) The thus-obtained crystals (1 g) were dissolved in methanol (25 mL), and a 4% aqueous KI solution (25 mL) and 17% sulfuric acid (5 mL) were added to the solution. The resultant solution was titrated with a 0.02M aqueous sodium thiosulfate solution. The free iodine content was found to be 5 ppm. Through ICP-basis total element analysis, Li, Na, K, Mg, Ca, Sr, Ba, Sc, Y, Ti, Zr, V, Nb, Cr, Mo, W, Mn, Fe, Ru, Co, Rh, Ni, Pd, Pt, Cu, Ag, Au, Zn, Cd, Al, In, Si, Sn, Pb, P, Sb, or S was not detected, and the Group 1 element content and the Group 2 element content were both 1 ppm or less. [Example 15]; In a 10-L reactor equipped with a reflux condenser, acetic acid (2,678 g), acetic anhydride (823 g), 2-methylbenzoic acid (700 g), iodine (502 g), a 70% aqueous iodic acid solution (299 g), and H-β-form zeolite (161 g) were placed. The mixture was allowed to react at a reflux temperature of 122°C for four hours. After completion of reaction, H-β-form zeolite was removed from the reaction mixture through filtration. A 10% aqueous sodium thiosulfate solution (200 g) and water (2,500 g) were added to the filtrate, and the mixture was cooled to 30°C. The precipitated crystals were collected through filtration, to thereby yield 1,204 g (after drying) of a product. Through HPLC (high-performance liquid chromatography) analysis of the collected crystals and the mother liquor, the following reaction results were obtained. 2-methylbenzoic acid: 97.0% (conversion) 5-iodo-2-methylbenzoic acid: 94.3% (yield) 97.2% (selectivity) 3-iodo-2-methylbenzoic acid: 0.7% (yield) 0.7% (selectivity) 5-iodo-2-methylbenzoic acid crystals: 89.6% (yield) 5-iodo-2-methylbenzoic acid: 99.7% (purity in crystals) The thus-obtained crystals (1 g) were dissolved in methanol (25 mL), and a 4% aqueous KI solution (25 mL) and 17% sulfuric acid (5 mL) were added to the solution. The resultant solution was titrated with a 0.02M aqueous sodium thiosulfate solution. The iodine content was found to be 5 ppm. Through ICP-basis total element analysis, Li, Na, K, Mg, Ca, Sr, Ba, Sc, Y, Ti, Zr, V, Nb, Cr, Mo, W, Mn, Fe, Ru, Co, Rh, Ni, Pd, Pt, Cu, Ag, Au, Zn, Cd, Al, In, Si, Sn, Pb, P, Sb, or S was not detected, and the Group 1 element content and the Group 2 element content were both 1 ppm or less.
References:
MITSUBISHI GAS CHEMICAL COMPANY, INC. EP1642881, 2006, A1 Location in patent:Page/Page column 6
52130-17-3
335 suppliers
$20.00/25g

133232-56-1
142 suppliers
$12.00/1g

118-90-1
533 suppliers
$13.00/1g
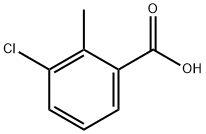
7499-08-3
189 suppliers
$8.00/10g
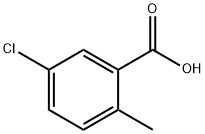
7499-06-1
180 suppliers
$5.00/1g

133232-56-1
142 suppliers
$12.00/1g

54811-38-0
406 suppliers
$5.00/5g
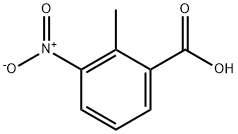
1975-50-4
467 suppliers
$18.00/25g

133232-56-1
142 suppliers
$12.00/1g
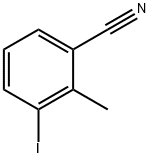
52107-66-1
29 suppliers
inquiry

133232-56-1
142 suppliers
$12.00/1g