
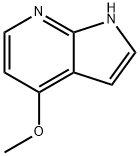
3-Iodo-4-methoxy-1H-pyrrolo[2,3-b]pyridine synthesis
- Product Name:3-Iodo-4-methoxy-1H-pyrrolo[2,3-b]pyridine
- CAS Number:928653-75-2
- Molecular formula:C8H7IN2O
- Molecular Weight:274.06
122379-63-9
147 suppliers
$5.00/5mg
![3-Iodo-4-methoxy-1H-pyrrolo[2,3-b]pyridine](/CAS/GIF/928653-75-2.gif)
928653-75-2
46 suppliers
$106.00/250mg
Yield:-
Reaction Conditions:
with iodine;potassium hydroxide in N,N-dimethyl-formamide at 20; for 0.916667 h;
Steps:
2.2 Preparation of 3-iodo-4-methoxy-1-tosyl-1H-pyrrolo[2,3-b]pyridine (1b)
4-Methoxy-7-azaindol (0.74 g, 5.00 mmol) and potassium hydroxide (0.70 g, 12.5 mmol)were dissolved in DMF (30.0 mL). A solution of iodine (1.28 g, 5.05 mmol) in DMF (30.0 mL)was added dropwise to the solution at room temp and the resulting mixture was stirred for45 min. After addition of a second portion of potassium hydroxide (0.70 g, 12.5 mmol), themixture was stirred at room temp for 10 min before a solution of p-toluene sulfonyl chloride(2.00 g, 10.5 mmol) in DMF (30.0 mL) was added dropwise. The resulting mixture was stirredat room temp for 2.5 h. After complete conversion (monitored by TLC) the reaction mixturewas poured onto ice water (150 mL). The resulting yellow precipitate was filtrated and driedin vacuo to give the desired product 1b (2.02 g, 94%) as a light yellow solid.
References:
Drieβen, Daniel;Biesen, Lukas;Müller, Thomas J. J. [Synlett,2021,vol. 32,# 5,art. no. ST-2020-B0180-C,p. 491 - 496] Location in patent:supporting information